Researchers from Flanders and Italy have developed a strategy to restore the function of an enzyme that is impaired in Parkinson’s disease. In an article published in Nature Communications, they demonstrate how antibody fragments can bind to the enzyme in the correct location to stabilise it and enhance its activity.
Parkinson’s disease is a neurodegenerative condition that causes a deterioration in motor skills. This results in tremors, movement difficulties and balance problems. Although ten million people worldwide suffer from the disease, there is still no cure, and the cause remains unknown in many cases. However, it is clear that mutations of the enzyme glucocerebrosidase (GCase), which breaks down specific lipids, play an important role in the disease. Disruption to the enzyme’s function causes waste products to continuously accumulate in cells, increasing the risk of developing Parkinson’s disease.
Now, two research groups, one led by Wim Versées at the VIB-VUB Centre for Structural Biology in Brussels and the other by Nicoletta Plotegher at the University of Padua, have developed a new strategy to protect and restore GCase function. They achieved this by binding nanobodies — small, stable fragments of special antibodies — to the enzyme with extreme precision. Versées: ‘The nanobodies stabilise the structure and function of the enzyme. We have also discovered some nanobodies that can restore the function of mutated GCase.’
Camelid antibodies
Mutations can cause GCase to unfold and become stuck during its journey through the cell, resulting in cell death. These adverse effects can be prevented by so-called ‘molecular chaperones’ that bind to the enzyme to protect or restore its shape and function. However, one problem with traditional molecular chaperones is that they can bind undesirably to the active site of GCase, causing it to lose its function.
Nanobodies are suitable candidates for use as molecular chaperones because they can be easily designed to avoid binding to the active site of the enzyme. These nano-proteins are fragments of unique antibodies that are found naturally in camelids. While human antibodies require multiple domains to bind, those of camels and llamas, for example, have only a single binding domain. ‘This domain can be produced and function independently, thus forming a nanobody’, says Versées. ‘Their simplicity makes them much easier to produce in large quantities than human antibodies. In addition, nanobodies have long loops which enable them to penetrate small recesses on the surface of proteins more effectively in order to bind there.’
The researchers developed several nanobodies that could bind to GCase. When they analysed their affinity and stability, they found that some variants prevented the enzyme from unfolding due to their binding properties. A few nanobodies also appeared to increase the enzyme’s activity.
Article continues below the image
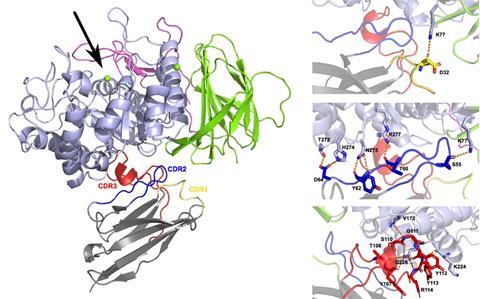
Binding affinity
Co-first authors Thomas Dal Maso (VIB-VUB) and Chiara Sinisgalli (University of Padua) carried out most of the experiments. While Sinisgalli investigated the action of the nanobodies in cells in Italy, Dal Maso focused on the biochemical characterisation of the different nanobodies.
Among other things, he measured the binding affinity between the nanobodies and GCase to find the right conditions. ‘We covered a surface with a type of nanobody and shone light through it’, he explains. ‘When the GCase in the administered liquid bound to these nanobodies, the refractive index of the surface changed, enabling us to determine the affinity.’ Interestingly, Dal Maso discovered a nanobody that showed very low affinity yet enhanced the activity of the enzyme the most. ‘Now we want to increase the affinity of this nanobody, which should further enhance its effectiveness.’
Barrier
Ultimately, the researchers hope to apply these results to a practical therapy. ‘But that’s not on the agenda yet’, says Versées. ‘First, we need to test many things in mouse models and patient cells in the lab before we can administer the nanobodies to patients.’
In addition, the blood-brain barrier, which is the boundary between the bloodstream and the brain, poses a major challenge. The protein fragments are too large to penetrate the barrier, which prevents them from reaching the brain cells where they are needed. Versées: ‘That’s why we are now looking for techniques that can shuttle the nanobodies into the brain, for example by having them carried by a harmless virus.’
Dal Maso, T., Sinisgalli, C. et al. (2025) Nat. commun. 16(4890), DOI: 10.1038/s41467-025-60134-4












Nog geen opmerkingen