In PNAS, biochemists from Utrecht University and their international colleagues present a new measurement method called FibrilPaint, which makes the growth of harmful protein clumps in the brain visible.
A major problem with diagnosing dementia is that it is often detected too late. By the time memory loss is detected, most of the brain damage has already occurred. The leading hypothesis for the cause of dementia and other neurodegenerative diseases, including Parkinson’s and Huntington’s disease, is the formation of amyloid fibrils in the brain. These thin, fibrous protein clumps can grow silently for years without the patient noticing. Existing techniques are nearly unable to detect these early changes.
This may now change, however. Researchers from the group of biochemist Stefan Rüdiger at Utrecht University, together with international colleagues, have developed a new measurement method that makes the growth of amyloid fibrils visible.
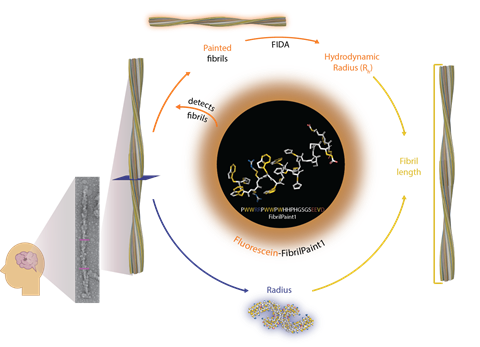
The technique, called FibrilPaint, uses a specially designed fluorescent peptide that binds to the protein strands with high affinity — effectively ‘painting’ them. This enables the presence of fibrils to be measured directly in blood or spinal fluid from patients.
‘Almost all central proteins in various neurodegenerative diseases, from Alzheimer’s to Huntington’s, form amyloid structures’, says Júlia Aragonès Pedrola, co-first author and biochemist at Utrecht University. ‘Because FibrilPaint does not bind to a specific protein, but to the fibrils themselves, it can be used to detect all of these conditions.’
Measuring molecules
Rüdiger and his colleagues were originally working on a different question: how certain proteins, known as molecular chaperones, prevent the aggregation of amyloid fibrils in a healthy brain. To investigate this, he sought a technique to measure the length of the fibrils. He eventually came across a recently developed microfluidic capillary technique called FIDA, which can be used to quantify the length of molecules. ‘It seemed perfect to us’, says Rüdiger. ‘However, we were faced with the problem that it could only be used to measure fluorescent molecules. So we set to work developing a fluorescent peptide that would bind only to the fibrils and nothing else.’
Amyloid fibrils consist of stacked strands of a repeating protein. The researchers successfully designed a fluorescent peptide that attaches only to these strands. It leaves monomers and other aggregates alone. The researchers are still trying to understand how it does this. ‘Although our measurements show that FibrilPaint binds to the fibrils with nanomolar affinity, we don’t yet have a clear understanding of exactly how it binds’, says Rüdiger. ‘We suspect that the peptide bridges the stacked layers and binds to multiple strands, which would explain the relatively high affinity.’

Sifting through peptides
The Utrecht researchers developed and synthesised the peptide in collaboration with international colleagues. ‘First, we screened a large set of potential peptides to find the one that bound best to amyloid fibrils’, says co-first author and Utrecht University biochemist Françoise Dekker. ‘We did this using Thioflavin T, a dye that gives a signal depending on the growth of amyloid aggregates. When a peptide binds to the fibrils, this affects the ThT signal.’
The researchers then used this information to identify a subset of four peptides that could bind to various neurodegenerative proteins, including the tau protein, which plays a significant role in Alzheimer’s disease. Through further optimisation steps to measure the length of the fibrils as accurately as possible, they identified the best candidate.
Clinical validation
Meanwhile, the researchers are working hard to bring FibrilPaint to the healthcare sector. Dekker is now the CEO of NeuroTidal Diagnostics, a start-up company that aims to apply the measurement method to clinical studies searching for drugs to treat dementia. Dekker: ‘We are currently screening a large group of patient samples with FibrilPaint.’
In addition to diagnosis, the peptide paint may also be used as a therapy. It serves as a molecule that attracts the cellular degradation system to clear away a specific protein (PROTAC). ‘After FibrilPaint binds to amyloid fibrils, it can signal to the cells’ machinery to break them down’, says Pedrola Aragonès. ‘Although we are still validating whether this degradation is successful, we have already demonstrated that the signalling works.’
Aragonès Pedrola, J., Dekker, F.A. et al. (2025) PNAS 122(44), DOI: 10.1073/pnas.2502847122












Nog geen opmerkingen