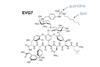
It’s been just over a year since C2W | Mens & Molecule first wrote about the super antibiotic EVG7. Now, the Leiden team has returned with a publication in Nature Communications showing that EVG7 selectively targets the harmful bacterium Clostridioides difficile without significantly affecting beneficial gut bacteria in a mouse model.
Even before the Science Translational Medicine paper was published, Professor of Biological Chemistry Nathaniel Martin presented some of the findings to Wiep Klaas Smits, a colleague from the Leiden University Medical Centre. ‘Wiep Klaas and his group are experts in C. diff’, says Martin, ‘and he offered to provide some real-world, patient-derived C. diff samples to test EVG7 on.’
As expected, the super antibiotic was highly effective since C. diff is a Gram-positive bacterium. ‘We consistently saw that EVG7 was ten times more potent against C. diff than the clinically-used gold standard, vancomycin’, explains Martin. While vancomycin does suppress the growth of C. diff, it also eliminates the so-called commensal bacteria that are beneficial to the gut. ‘In the absence of protective commensal bacterial even a trace of C. diff spores can lead to a relapse infection that could be even worse than the original.’ This relapse, or return of the infection, is something that many patients experience after being infected with this bacterium.
Curative effect
Martin and his team next evaluated EVG7 in a mouse model of C. diff infection developed by Casey Theriot at North Carolina State University, and it ‘worked beautifully’. In the model, mice treated with vancomycin usually experience a relapse after two weeks, but this was not the case with EVG7; it seems to have a lasting effect.
This observation is particularly exciting, explained Martin: ‘in the case of patients treated with vancomycin, they often have to take multiple rounds of antibiotics due to infection relapse, which is connected to a higher mortality rate. The finding that EVG7 provides a curative effect after a single treatment and with no indication of relapse is very encouraging.’
However, why is EVG7 more active against Clostridium than the beneficial commensal bacteria? ‘That’s a good question, and we still don’t have a definitive answer’, Martin acknowledges. ‘It may be EVG7’s structural modification that allows it to bind more effectively to C. difficile than to commensals, but this would require deeper mechanistic studies to confirm.’
Broad potential
Martin hopes that this paper will generate interest in EVG7 within the community. ‘At the moment, we’re quite active in conducting a number of preclinical studies. Together with Elma Mons, the postdoc leading the project in the Martin Group and Coen van Hasselt from the LACDR, we received a NACTAR grant from NWO to investigate how to dose the compound most effectively, which is crucial for approaching human trials.’
Alongside some other studies into biodistribution and toxicology, Martin and his colleagues are slowly building a strong case for the effectiveness and safety of EVG7. While the initial clinical indication to be pursued for their antibiotic will likely be acute bacterial skin and skin structure infections – ‘ABSSSIs, a common first indication for new antibiotics’ – the present study demonstrates its broader potential. ‘In the event that EVG7 is approved for an indication like ABSSSIs, it will pave the way for its evaluation in the treatment of other types of infections. Our recently published studies demonstrating its effectiveness in treating C. diff may therefore provide a rationale for using EVG7 to treat such infections in the future’, says Martin. ‘All in all, we’re compiling a compelling list of all EVG7’s advantages over vancomycin.’
Mons, E. et al. (2025) Nat. Commun. 16, DOI: 10.1038/s41467-025-64067-w











Nog geen opmerkingen