A spectacular image of an electrocatalyst that exhibits spectacular behaviour. During CO2 reduction, this combination of tin particles on a nanotextured carbon support manages to improve its performance. The secret: particle breakdown.
The search for ways to efficiently utilise CO2 as a carbon-feedstock is in full swing. Finding new catalysts to reduce CO2 is a key element of many of these projects. In ACS Applied Energy Materials, researchers at the universities of Nottingham and Birmingham now present a combination of tin particles on a nanotextured carbon fiber support as the ‘new kid on the block’ in electrocatalysis.
[Article continues below]
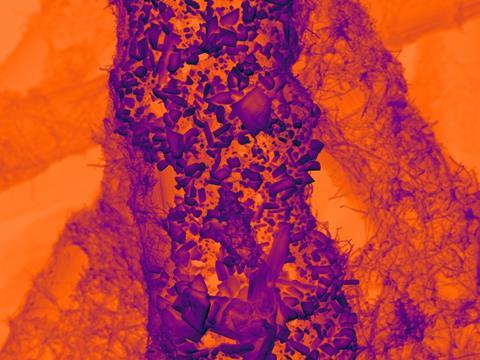
According to the team, the interactions between their catalyst’s graphitised carbon fibers and the tin particles ensure the observed high performance. In the paper’s accompanying press release, Madasamy Tanghamutu, research fellow at the University of Notthingham, states: ’A successful electrocatalyst must strongly bond to the CO2 molecule and efficiently inject electrons to break its chemical bonds. We developed a new type of carbon electrode that incorporates graphitised nanofibers with a nanoscale texture, featuring curved surfaces and step edges, to enhance interaction with tin particles.’
Another key feature of the catalyst concerns the surprising increase in activity during use. ’We can assess the performance of the catalyst by measuring the electrical current consumed by the reacting CO2 molecules. Typically, catalysts degrade during use, resulting in decreased activity. Surprisingly, we observed the current flowing through tin on nanotextured carbon increased continuously over 48 hours. Analysis of the reaction products confirmed nearly all electrons were utilised to reduce CO2 to formate, boosting productivity by a factor of 3.6 while maintaining nearly 100% selectivity’, explains first author Tom Burwell of the University of Notthingham.
Electron microscopy analysis of the catalyst revealed that the tin microparticles break down to nanoparticles as small as 3nm during CO2 reduction. And that results in this remarkable self-optimisation behaviour. Burwell: ’We found that smaller tin particles achieved better contact with the nanotextured carbon of the electrode, improving electron transport and increasing the number of active tin centres nearly tenfold.’
Tom Burwell, et al., In situ transformation of tin microparticles to nanoparticles on nanotextured carbon support boosts the efficiency of the electrochemical CO2 reduction, ACS Applied Energy Materials (2025), doi:10.1021/acsaem.4c02830












Nog geen opmerkingen