For organic chemists (in training), the question of how to determine whether a chemical reaction follows the SN2 or E2 route is timeless. A group in Amsterdam summarized its 25 years of research in Chemistry: A European Journal, offering concrete, simple concepts to overcome this dilemma.
Organic chemistry has its challenges. Where does a molecule attack? Does a molecule react as a base or a nucleophile? Does a reaction proceed via E1, SN1, or SN2? Of course, there’s also the question of the competition between SN2 vs. E2 reactions. Thomas Hansen and Pascal Vermeeren, assistant professors at Free University Amsterdam, began researching the latter topic five to six years ago as part of their Ph.D. studies. The publications resulting from their work are now summarized in this Concept article.

A special time
‘This project also marked the beginning of our friendship, and we are still working together’, says Vermeeren. ‘It’s a great moment to close this chapter and summarize everything.’ Hansen agrees from the same office: ‘Now that we have started our own lines of research, this is the icing on the cake of all our adventures.’
It was an especially meaningful time for the two young researchers because they did most of the work during the pandemic. ‘As theorists, we were able to continue working relatively normally, so our days were filled with video calls, writing, and doing calculations.’
The TheoCheM group, where Hansen and Vermeeren conducted their research, has a philosophy of ‘using the activation energy model to gain insight into how reactions work and translating that into insights that the experimentalist can use immediately’, says Hansen, an experimental chemist by training. ‘That’s what makes this research so great. It goes back to concepts that all chemists can understand.’ In this way, they provide tools that are easy for the reader to understand.
Deformation
The main concepts from the article are characteristic distortivity, acidity of the transition state, and intrinsic and actual nucleophilicity. ‘For example, you see that the distortivity of the reactants differs significantly’, Vermeeren explains. ‘Distortivity requires energy, which molecules generally want to avoid.’
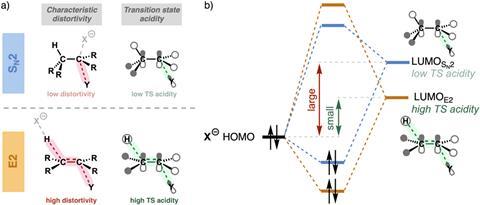
In an SN2 reaction, one bond is broken. However, in an E2 reaction, two bonds are broken. ‘Therefore, more energy is required’, Vermeeren continues. ‘At the same time, however, distortivity also affects the electronic structure. The LUMO of the substrate decreases in energy, improving the interaction with the Lewis base. If the base is strong enough, it becomes more favorable to follow the E2 pathway.’
According to Hansen, it’s quite a unique situation. ‘You have two reaction pathways present at the same time. For both pathways, if the Lewis base becomes weaker, the reaction barrier increases. But not at the same rate!’ The pathways cross at a certain point. With a strong Lewis base, E2 is more favorable; with a weak base, SN2 is more favorable.
Stumbling block
The paper was written so that all chemists, from students to theorists, could benefit from it. ‘We hope that it will be included in courses and lectures to make this concept more understandable’, says Vermeeren. Hansen adds, ‘We have really done our best to make the figures as clear as possible, almost textbook-like. Organic chemistry remains a stumbling block for many, so we tried to make it as easy as possible.’ They did not shy away from complicated and complex systems or the effects of solvents in doing so. ‘We wanted to demonstrate the relevance of this work.’
These types of competitive reactions are inherently complex, and providing conclusive guidance takes years of research. ’Introducing carbocations into the models, as in an E1 or SN1, complicates matters’, says Hansen. ’But other reaction competitions are being extensively researched. Jeroen Codée is tackling the SN1-SN2 competition, and there are still some hurdles to overcome.’ The approach remains the same throughout: ‘A calculation and an experiment!’ laughs Hansen.
Vermeeren, P., Hansen, T., et al., (2025). Chem. Eur. J. e202501810, DOI: 10.1002/chem.202501810












Nog geen opmerkingen