With the high-tech laser facility of HFML-FELIX, researchers succeeded in capturing a catalyst ‘in the act’ of catalysing a Michael addition, as they show in The Journal of Physical Chemistry Letters.
The processes that occur during catalytic reactions, particularly at the active site or during the transition state, are often still shrouded in mystery. However, researchers from the University of Amsterdam (UvA), the HFML-FELIX research facility and Radboud University have managed to uncover the reactive structure of 1-(2-nitroethyl)naphthalene (a nitroolefin) to Takemoto’s catalyst as part of the well-known Michael addition of this compound to malonates.
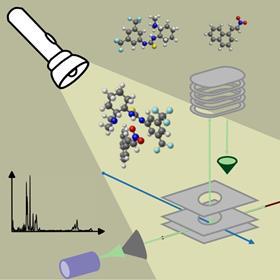
‘I was working on these chiral reactions using VCD spectroscopy and looking for their reactive intermediates, but so far we were unsuccessful’, says Wybren Jan Buma, Professor of Molecular Spectroscopy at the UvA. ‘It just so happens that I also have a background in molecular beams, so I thought, “Let’s give that a try.”’ He contacted his connections at HFML-FELIX where he also has a part-time appointment, the state-of-the-art IR laser facility in Nijmegen, and applied for beam time with postdoc Sander Lemmens to trap the intermediates in the gas phase .
Complex
It took some time for the project to get underway, as the experiments were very challenging. However, thanks to the expertise of one of the staff members of HFML-FELIX, Piero Ferrari, they succeeded. ‘The setup works well when you want to study a single molecule’, Ferrari explains. ‘But in this case, we needed to develop specific conditions to simultaneously capture two or three species in the beam. They couldn’t just be flying around; they also had to form a complex.’ Additionally, the complex had to be stable for long enough to enable spectroscopy of the intermediate states.
Another challenge was getting the complex into the beam. ‘Usually, you create a vapour of your compound by heating it or using laser desorption’, says Buma. ‘But in our case, the molecules are solid or liquid, so we had to combine several vaporisation techniques simultaneously. Resolving that required some serious Fingerspitzengefühl.’ At one point, Ferrari adds, ’we were surprised it actually worked’.
Pinpoint
The team conducted the experiments sequentially using the aptly named Bundle Machine (BUMA). After some initial struggles, they found conditions in which the catalyst was visible in complex with one reactant. ‘We then noticed that the conformation of the catalyst was different from that described in the literature’, says Ferrari. Buma continues, ‘It had to do with the reactive state. Just as with liquids, the catalyst adopts a different conformation when reacting with the substrates.’ This enabled them to pinpoint exactly what was happening with the hydrogen bonds at the catalyst.
Seeing the catalyst in action is quite special. ‘Normally, the reactive intermediate is a fleeting state that is extremely difficult to observe’, explains Buma. ‘But we were able to trap the intermediate just before it reacted, which is really unique.’ Although the researchers demonstrated this using a classic Michael addition, the same principle applies to any catalytic reaction.
Article continues below image

‘This provides lots of information about the geometry and intermolecular interactions, such as hydrogen bonds’, adds Ferrari. ’Plus, we can do it with molecules in neutral states.’ So far, these kinds of analyses had only been done on reactions involving charged species which are much easier to create and manipulate. ‘This opens up many possibilities, not just for organocatalysts, but also for metal-based ones in numerous reactions.’
Calculations
While Buma has nothing but praise for the facility, he also clarifies that molecular dynamics calculations are necessary too. ‘FELIX is superb, but one complicating factor is that when you measure a spectrum, you only get a general idea of the structure. To pinpoint it more precisely, calculations are required, and the larger the molecules and clusters, the more difficult it becomes. Piero did a tremendous amount of work on this part of the project.’
‘It takes a lot of time’, adds Ferrari. ‘There are many atoms and conformations to consider, both separately and in complexes. It’s a huge space to cover.’ However, calculations should always go hand in hand with experiments, especially if they are this complicated. ‘It’s unusual to study these clusters of three large molecules’, Buma continues. ‘This is the first time someone has been stupid enough to try it, but it has turned out better than we expected!’
According to Buma, this project opens up applications in a wider range of catalysts. But there’s more: ‘From the molecular beam point of view, this creates a whole new area of research. Now that we’ve shown it’s possible, I’m sure others will follow.’
Ferrari, P., Lemmens, A.K. & Buma, W.J. (2025) J. Phys. Chem. Lett. 16(24), DOI: 10.1021/acs.jpclett.5c01093












Nog geen opmerkingen