By manipulating the kinetics of sterol binding, an important but also highly toxic antifungal drug could become a lot safer for patients, US-based chemists report in Nature.
Amphotericin B is a last-resort antifungal drug that doctors turn to when treating patients with drug-resistant fungal infections. However, amphotericin B is also notorious for its very harmful side effects; the drug is particularly toxic to the kidneys. A team from the University of Illinois at Urbana-Champaign, led by chemist Martin Burke, have now succeeded in modifying the drug’s molecular structure to counter its toxicity, while maintaining its efficacy.
Yearly, 1.5 people worldwide die from fungal infections, compared to 700,000 people that die from an infection with resistant bacteria and over 600,000 from malaria. If antibiotics fail to treat a bacterial infection, there are often still alternatives available. When it comes to drug-resistant fungi, the choice is very limited. This is partly because resistant fungal infections are much less studied than antibiotic-resistant bacteria.
Dilemma

Since 1960, amphotericin B is one of the few options left, when other drugs fail. It is broad-spectrum antifungal and resistance rarely occurs. Important advantages, but there is also a major drawback: the drug is very toxic, especially to the kidneys. ‘During my internships, we also called the drug ampho-terrible,’ Martin Burke explained in a Nature podcast. ‘Immediately after administering the drug to the patient, a very unpleasant fever reaction occurs. Even worse, in the long term it is very toxic to the kidneys.’ Especially in mucor- or rhizopus-infections, high doses are needed. This presents doctors with a difficult dilemma: a high dose causes severe kidney damage, but when to dose is too low, the fungus will survive.
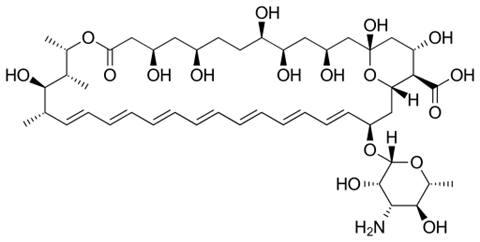
Burke and colleagues found that amphotericin kills fungal cells by forming sponge-like aggregates on the cell surface that remove sterols from the cell membrane. Those sterols are essential for the membrane. Without them, the fungus dies. Human cell membranes contain cholesterol, which is very similar to the fungal sterols. In humans, amphotericin removes cholesterol from the membrane in a similar way, Burke’s research shows. Hence the toxicity in patients.
The team discovered this by examining analogues of amphotericin B that exhibited structural variations at different sites in the molecules. This allowed them to evaluate how structural changes affect the biological activity of the drug. By subsequently testing these analogues in human kidney cells and in mice, they saw that renal toxicity is caused by the binding of amphotericin B to cholesterol in the kidney cell membrane.
A little weaker
That provided clues to circumvent renal toxicity. ‘Once we knew how the drug destroys kidney cells, we needed to change that, so that it would only kill fungal cells,’ Burke said in the podcast. High-resolution NMR structures of amphotericin B with and without bound sterol molecules showed that that amphotericin binds strongly to fungal sterols. Much stronger than the binding to human sterols. That provides them with a lead. Burke: ‘What if we can make that bond a little less strong so that the drug can no longer bind cholesterol, but the bond with fungal sterols only becomes a little weaker?’
The first modified amphotericin analogue proved that this could be done. There was just one problem: potency was seriously diminished. The fungus produced new ergosterol faster than the drug analogue could remove it. The researchers therefore made another modification to the structure that changed the variant’s kinetics to ensure even faster sterol removal. This resultated in analogue AM-2-19, named after first author Arun Maji. Finally, they tested AM-2-19 in vitro against 500 different fungal species. Burke: ‘It turned out to be very potent.’ And they conducted further toxicity studies in human kidney cells and in mice with fungal infections. AM-2-19 proved effective, without being toxic.
Impressive
‘I find the design-by-structure chemistry of this study very impressive,’ responds clinical pharmacologist Roger Brüggemann, who researches resistant fungal infections at Radboudumc in Nijmegen. ‘It is very complete because they elucidated the structure using NMR and investigated the interaction with the sterols, and managed to manipulate the binding, and also performed lab research, animal studies and in vitro studies on human cells.’ Brüggemann further points out that the ergosterol removal mechanism occurs in more antifungals from the same family, so these modifications might be useful for more antifungal drugs.
Why the second modification of AM-2-19 provided the desired effect, the researchers do not yet know. Follow-up research is needed, but for now Burke’s team is happy with the result. They are so convinced of the potential of their work, that Burke has founded a company, Sfunga Therapeutics, to further develop the molecule. Recently, the first clinical trials have begun. Brüggemann: ‘I am very curious to learn the results. A drug with this effect without the toxicity is exactly what we are looking for.’
Nature podcast, November 8 2023, with contributions from Martin Burke












Nog geen opmerkingen