A team from Utrecht University functionalises the C-H-bonds in polyolefins using just UV light without the need for solvents or catalysts, they report in JACS.
What came first, posttranslational modifications (PTMs) of proteins or post-polymerisation modifications (PPMs)? PTMs are of course as old as life itself, but the first cases of PPMs even predate the full insight into amino acid structures. ‘Those communities evolved very separately’, says Pieter Bruijnincx, professor of Sustainable Chemistry & Catalysis at Utrecht University. ‘Though they might seem similar, you’re talking about two completely different worlds of molecules.’
Feedstock
The field of PPM seeks to introduce functional groups in polymers. ‘In this work, we focus on introducing oxime-groups in polyolefins’, says assistant professor Arnaud Thevenon-Kozub from the same university. ‘Polyolefins only contain C-H-bonds, which are notoriously difficult to functionalise.’ In the past, some people were already able to introduce oxime-groups, but it required multiple steps to get there.’ In a publication in JACS, the team of Bruijnincx, Thevenon-Kozub, Maartje Otten and colleagues showed a chemoselective single step photochemical oximation of polyethylene.
‘There has been a renaissance of this field over the last twenty years’, says Bruijnincx. ‘But what was still lacking were robust methods to introduce reactive handles that are easy to apply. Maartje did exactly that.’ Otten, a PhD student supervised by both Bruijnincx and Thevenon-Kozub , adds: ‘In general, post-polymerization modification is difficult without a reactive handle. Though olefins only have C-H-bonds that are hard to functionalise, they are the most ubiquitous type of polymer, so we thought, why not make a feedstock out of it?’
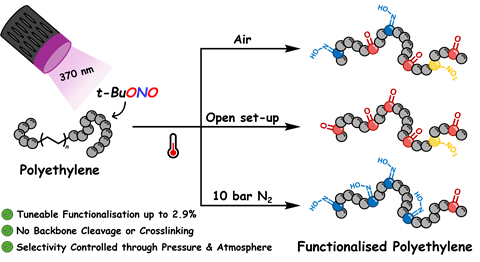
Blessing
But research wouldn’t be research if it lacked surprises. ‘We saw a change in chemoselectivity’, says Otten. ‘Instead of the oxime, we observed ketone functional groups as the main species after the reaction, and we had no clue why or how this happened.’

Usually, Otten ran the reaction overnight. One time, she did it during the day and she discovered that the reaction mixture became intensely blue (see picture). ‘When we looked into it, we suspected that it might be caused by a second consecutive radical reaction at the installed oxime group.’ This was confirmed by EPR spectroscopy. ‘UV light is both a blessing and a curse’, Otten continues. ‘It does create the oxime, but when left alone, the oxime keeps reacting towards the ketone.’
Luckily, the team found a way to take back control over the reaction. ‘When you pressurise the reaction mixture with nitrogen gas, it chemoselectively keeps the oxime, which was our main target’, says Otten. However, if you would like to get ketones, you could let the reaction run free so that all gasses formed can escape. ‘Our method works well if you want flexibility, but there are better ways to create just ketones.’
Stepping stone
Remarkably, despite it being radical based chemistry, the backbone of the polymer stays intact. Thevenon-Kozub sees interesting possibilities for recycling of plastics. ‘When you react the oximes towards amides for example, you could make a mixture of incompatible waste polymers more compatible with each other, which helps recycling.’
Another application for oxime-functionalised polyolefins is to improve adhesive properties, says Otten. ‘It makes the polymer more polar and thus more compatible with other polar compounds.’ The oxime can also be a stepping stone toward other functionalities. Thevenon-Kozub: ‘By varying the groups, you can either keep the polyolefin properties or add others. The reactive handles also helps when you want to cleave the polymer at certain points to retrieve the building blocks.’ In other words, you create functional use advantages as well as potential end of life advantages, Bruijnincx adds.
Otten, M. et al. (2025) JACS 147(26), DOI: 10.1021/jacs.5c05212













Nog geen opmerkingen