In the European Journal of Organic Chemistry, researchers from the VUB and the University of the Free State (South Africa) present a new fluorescent peptide hydrogel with easily adaptable sequences. This offers possibilities for targeted, modifiable drug delivery systems.
Hydrogels are attractive biomaterials for the controlled delivery of drugs due to their unique properties. When subjected to pressure, the gel becomes liquid enough to be injected subcutaneously; however, it forms a gel again almost immediately afterwards. The gel then erodes slowly, releasing the stored biologically active components gradually. Researchers at the Vrije Universiteit Brussel (VUB), including chemists Steven Ballet, Charlotte Martin and Ulrich Hennecke, are developing hydrogels consisting of natural peptides that are easily degradable in the body.
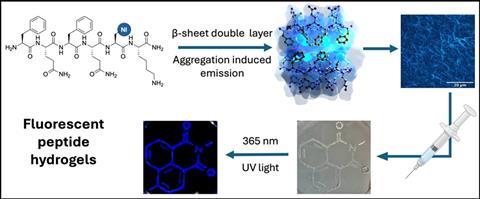
To develop these peptide hydrogels, the VUB researchers synthesise modified amino acids with specific properties. These amino acids are then placed in a peptide sequence known to assemble into a hydrogel. They developed a new amino acid with a little help from serendipity. This amino acid is based on 1,8-naphthalimide, and it forms a fluorescent peptide hydrogel with enhanced emission after aggregation. Professor of organic chemistry Steven Ballet explains: ‘Our peptide sequence previously consisted of phenylalanines that self-assemble into beta-sheet structures. However, when we altered the side chains of the amino acids, we found that the gels became fluorescent.’
By chance
The peptide sequence that the researchers are working with consists of amino acids with hydrophobic benzene rings on the same side of the chain. These aromatic side chains associate with each other, causing the formation of peptide fibres and, consequently, a stable hydrogel. Originally, Ballet and his colleagues were looking for ways to increase stability further and create stronger fibres. ‘We wanted to replace the aromatics with larger hydrophobic polycyclic systems to create even more stability’, says Ballet. ‘Quite by chance, we discovered that some of our replacements caused the chains to become fluorescent.’
This chance discovery worked out well for the researchers. Their main goal is to develop controlled delivery systems with modifiable properties. With the added property of fluorescence, the gel can be seen after subcutaneous injection. ’This is useful for imaging if we want to investigate where the gels end up after injection’, says Ballet. ‘Now we can observe in vivo for ten to twenty days to see how long the gel remains at the injection site and how quickly it erodes. This gives us the insights we need to develop better systems.’
Enhanced emission
The researchers synthesised their amino acids using palladium-catalysed coupling reactions, among other methods. By embedding these amino acids in the peptide sequence, they were able to maximise the fluorescence. The formation of beta-sheet structures determined the orientation of the luminescent side chains, causing the emission to increase after aggregation.
’The production of the amino acids was the most labour-intensive part of this study’, says Ballet. ’It was uncertain what material properties would emerge. But that is also the beauty of organic chemistry: you can keep adapting molecules to suit your needs. Once you have a lead, you can continue to modify the structures to refine the properties of the peptide chain.’
Antibodies
As they work towards controlled delivery systems, the researchers hope to gain even more control over their biomaterials. For example, their current peptide gels release the active ingredient within the first three to seven days after injection, making them less long-lasting than gels containing synthetic polymers. Ballet explains, ’Thanks to the modularity of our peptides, however, we can develop new systems relatively quickly by adjusting the structures. Our aim is to create stronger materials that break down more slowly in the body.’
In addition, the researchers intend to conduct further research into the compatibility between the active ingredients and the material properties of the hydrogel. They have already demonstrated that their hydrogel is suitable for releasing small molecules, peptides, and nanobodies (smaller variants of antibodies). ‘But that does not cover the entire spectrum of possible medicines’, says Ballet. ‘We are now looking at whether our system can also release large antibodies. The beauty of our system is that, instead of using synthetic polymers with limited adaptability, our gel is versatile and can be converted into a ‘friendlier’ environment for therapeutic proteins.’
Jolien Bertouille, Adelaide R. Mashweu, et al., Fluorescent β-sheet-based peptide hydrogels with aggregation-enhanced emission properties, EurJOC (2025), doi:10.1002/ejoc.202500730












Nog geen opmerkingen