Using a metal-organic framework (MOF), researchers in Leuven have developed a sensor that can distinguish gas molecules based on speed, as reported in Nature Communications.
The Nobel Prize in Chemistry is still fresh in everyone’s memory, especially among researchers studying metal-organic frameworks (MOFs). ‘We were actually expecting it last year’, says Margot Verstreken, a postdoctoral researcher in Rob Ameloot’s MOF research group at KU Leuven. ‘Though it didn’t happen then, we’re even happier that it has now.’
According to Verstreken, there is a good chance that the Nobel Prize will give the materials the boost they need. ‘The timing is perfect for us because we are just about to bring a few research projects to market.’ She laughs as she recalls how the group initially avoided the technical term ‘MOF’ — ‘we talked about sponge-like materials’ — but using it would actually help now. ‘There aren’t many MOF groups in Belgium, so quite a few people have already approached us. For example, the Flemish public broadcaster has come to film our lab.’
Speed camera
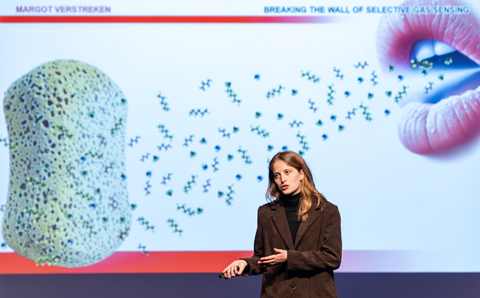
Coincidentally, the week before the Nobel Prize ceremony, Verstreken, Ameloot and their colleagues published a paper on how MOFs can be used to make a sensor that can distinguish volatile organic compounds based on their kinetic properties. ‘We use the comparison with a speed camera’, Verstreken explains. ‘We measure how fast gas molecules move through the nanopore structure of our MOF materials.’
Since MOFs consist of multiple components, the path that a molecule must travel can be adjusted by adding chemical functionality. ‘This makes it controllable and provides selectivity in gas sensing’, Verstreken continues. ‘Every molecule looks different and therefore has its own speed. This allows us to detect them on a finer scale.’
The process works as follows: the MOF material is brought into equilibrium with a mixture of gases that adsorb at room temperature. Increasing the temperature by tens of degrees in a short period of time then creates a different equilibrium, pushing the molecules out through the lattice. A sensor tracks the decrease in those molecules, detecting which ones have come out and when. You can study all kinds of gases in this way because you have complete control over the material and can focus solely on the movement. The sensor architecture can also be varied.
Please continue reading below the image

Water interference
With a hundred thousand different MOF structures now known, how do you choose the right one? ‘It depends partly on the gases we want to measure’, says Verstreken. ‘For us, it was important to be able to incorporate the MOF into the sensor as a thin film. We opted for the well-known MOF ZIF-8 and demonstrated that it is possible to easily distinguish between alcohols, alkanes and ketones, such as propanol and butanol.’
One problem that many MOF applications face is water, which often has a concentration a thousand times higher than the molecules you actually want to study and can interfere with the process. However, Verstreken and her colleagues do not encounter this issue with their sensor. ‘By designing the MOF properly, water can move through the pores very quickly’, she explains. ‘With the temperature jump in our sensor, all the water has already evaporated by the time the target temperature is reached. So for us, there is no difference between dry and humid conditions.’
Luxury problem
The sensor already outperforms a ten-part commercial sensor. But is there a clear path to practical application for this new technology yet? ‘That is the question we are now asking ourselves’, says Verstreken. ‘The fundamentals have been proven; we know how and when it works. But what is the most efficient application for the market? What problems need to be solved and how can they be solved?’ As well as its use in process monitoring, they are considering enquiries from the public sector concerning drugs, explosives and air quality.
The coming year will be dominated by marketing a single application. ‘We are busy working on the demonstrator. In my role as tech transfer postdoc, I am currently focusing on getting our technology out of the lab.’ The team is facing a luxury problem, says Verstreken. ‘There are many possibilities, which means that choices have to be made. We are still open to questions and collaborations, and we want to talk to everyone.’
Falling Walls
The Ameloot team wanted to share their results with the general public. Verstreken therefore threw herself into science communication and entered the Falling Walls competition, winning the national round. ‘It forced me to step outside my comfort zone, but I now recommend that everyone participates, as communicating well is incredibly important.’
Verstreken believes that there is a significant disconnect between academic research and how it is portrayed in the media. ‘We sometimes speak a different language, but competitions like this help to make that social aspect more visible.’ The team is now preparing for the final in Berlin in November, where they will compete against a hundred other national winners at the Falling Walls Science Summit.
Matavž, A., Verstreken, M.F.K. et al. (2025) Nat. Commun. 16, DOI: 10.1038/s41467-025-64199-z












Nog geen opmerkingen