Researchers at UMC Utrecht and AMOLF have discovered that two proteins influence each other on the same immune cell to adjust inhibitory signals. They present this unexpected mechanism by which immune cells adapt their behaviour in Science Signaling.
At the end of the last century, immunologist Linde Meyaard discovered LAIR-1, an important inhibitory receptor found on human mononuclear white blood cells. When activated, LAIR-1 inhibits the immune response to prevent the cells from causing damage. This has led to the receptor becoming a key target for cancer therapies.
Meyaard and her team at UMC Utrecht are still investigating the precise way in which LAIR-1 functions. It was expected that the activity of the receptor would depend primarily on its expression. However, working alongside the team of biophysicist Kristina Ganzinger from AMOLF, the researchers have now discovered that this is not the case. The MARCO protein, which is present on the surface of certain immune cells, can bind to LAIR-1 when both are located on the same cell — a process known as a ‘cis interaction’. Meyaard explains, “MARCO binds to this receptor in the same way as the LAIR-1 ligand, collagen. For this reason, we assumed that MARCO would activate LAIR-1.’ Immunologist Saskia Vijver, co-first author of the study, adds, ‘We wouldn’t have expected MARCO to actually inhibit the action of LAIR-1. This cis interaction, with regulation on the same cell, has not been seen before in this type of receptor.’
Brake on the brake
Before making this discovery, the researchers studied the trans-interaction between MARCO and LAIR-1. To do this, they expressed the proteins separately on different immune cells. When the two came together, they observed that MARCO stimulated the action of LAIR-1. ‘However, when we searched databases to find out where the proteins are expressed in the body, we discovered that MARCO is mainly present on cells where LAIR-1 is already present,’ says Meyaard. ‘So our in vitro study only reflected part of the reality. We also observed that tumours have a greater number of cells expressing both proteins.’
The researchers therefore decided to study these tumour-associated macrophages in vitro. Meyaard explains, ‘When MARCO is present on the same cell as LAIR-1, it does not cause an inhibitory signal; rather, it prevents the inhibitory signal caused by the original ligand.’
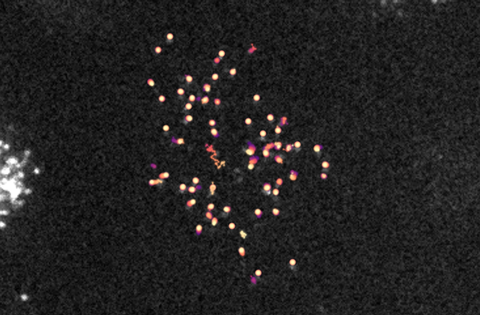
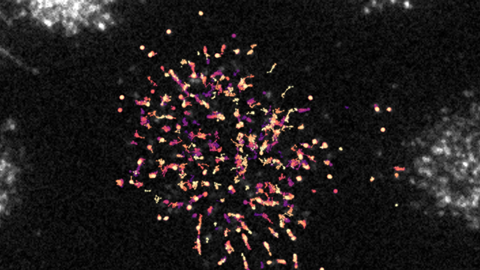
Looking at movement
To visualise this interaction, the researchers at UMC Utrecht collaborated with Ganzinger’s group at AMOLF. Using advanced microscopy techniques and tracking software, they were able to track MARCO and LAIR-1 as individual proteins across the surface of the cell. “To do this, we labelled both proteins with different fluorescent markers,” says Ganzinger. ‘By zooming in on the cell’s plasma membrane with our microscope, we were able to track both fluorescent receptors and their separate movements.’
The researchers discovered that MARCO moves very slowly while, when it occurs alone on a cell, LAIR-1 moves quickly. Ganzinger: ’But when both are expressed on the same cell, we observed that LAIR-1 moves at the same speed as MARCO, indicating a direct molecular interaction. When we then used antibodies to block this binding, LAIR-1 accelerated again.”
New avenue
This discovery provides insight into how immune cells regulate themselves. Rather than relying solely on external signals, MARCO’s functioning demonstrates how immune cells also influence themselves when they apply the brakes. Therefore, understanding exactly how these interactions work is of great importance for the development of immunotherapies against cancer.
The researchers have now discovered another binding partner for LAIR-1 that occurs on the same cell. For the time being, it appears that LAIR-1 is responsible for blocking this other receptor. The collaboration between UMC Utrecht and AMOLF continues in search of the precise mechanism of these interactions. Meyaard: “The fact that these types of cis interactions can be so decisive is a revelation to me. Suddenly, the self-regulation of cells by these types of receptors is a possibility. This opens up a whole new avenue for research.’
Singh, A. et al. (2025) Science Signaling 18(916), DOI: 10.1126/scisignal.ado2768












Nog geen opmerkingen