According to Ulf Hanefeld and Frank Hollmann of Delft University of Technology, being open to the unexpected is one of the most important qualities of a scientist. It was this attitude that led them to discover an enzymatic reaction that was previously thought impossible: transesterification in water.
Alcohol oxidases, which oxidise alcohol groups, are not usually associated with transesterification. So it came as quite a surprise when Delft professor Frank Hollmann observed ‘new’ peaks on a gas chromatography spectrum. ‘This happened during a collaboration with researchers from Guangzhou, with whom we wanted to expand the substrate scope of alcohol oxidases. The GC sample turned out to contain traces of esters, which you would never expect with alcohol oxidases.’
Professor Ulf Hanefeld, head of the same biocatalysis department as Hollmann, explains why this is so special: ‘Alcohol oxidases are redox enzymes, so the underlying mechanism works with electron transfer. The reaction we are now seeing, transesterification, works on a completely different principle. This is therefore a huge step forward. Mentally speaking, it’s almost impossible to comprehend.’
Side reaction
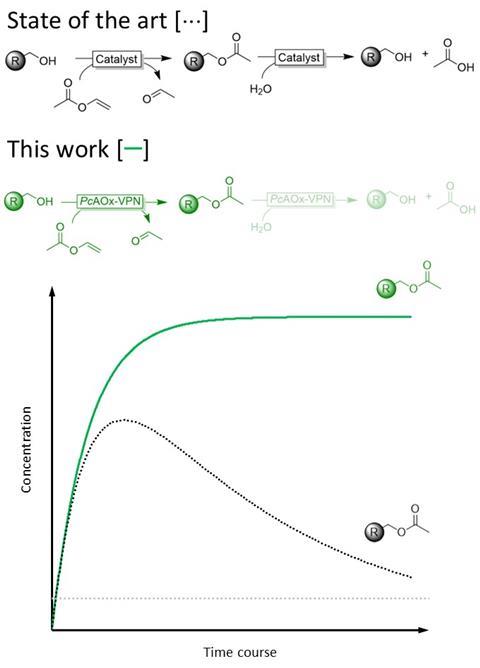
The surprises didn’t end there. ‘The transesterification was already impressive,’ says Hollmann, ‘but when we investigated the mechanism more closely, we saw that no hydrolysis takes place, which is normally the most significant side reaction.’
For years, Hanefeld had submitted project proposals – unfortunately without success – to explore this very idea. ‘So when Frank told me, I didn’t want to believe it at first,’ he says with a laugh. ‘I had already researched enzymes that suppress hydrolysis, but that side reaction was never completely eliminated. Even with enzymes that are already in use, you always see about ten per cent hydrolysis.’
It had been Hanefeld’s dream even before he started working as a researcher. ‘When someone suddenly says after thirty years that this dream has come true, you’re sceptical. Frank had to show me lots of experiments before I believed it.’
Thorough work
Although Hanefeld was eventually convinced, it was still quite difficult to publish the work. Hollmann said, ‘The editors often said, “The work shows improvement, but it’s not fundamentally new.” But we were persistent and persevered.’ And it worked: Nature Communications was eventually persuaded.
’That’s also thanks to the thorough work done by our Chinese colleagues. You can struggle against it, but if you strictly adhere to the test results and abandon the idea that ‘this is not possible’, it’s obvious.’
Now, (trans)esterification is already widely used in the food industry, for example, where mixtures of fats are treated. ‘You don’t work with water there, so our finding is not an asset for that industry,’ Hanefeld continues. ‘But it can play a role in the selective synthesis of esters in the pharmaceutical industry, for example. Many of the pills we take have to be water-soluble, and transesterification in water with an enzyme could be very helpful.’ However, a lot of research is still needed before that happens. ‘Which esters can the enzyme produce, and what about stereo- and regioselectivity? Once that is clear, the applications will follow naturally.’
More exciting
Hollmann and Hanefeld also want to emphasise that as a scientist, you have to be ‘open to the unexpected’. Hollmann: ‘In gas chromatography spectra, look not only at the expected peaks, but also at the unexpected ones. The first time we saw the ester peaks, they were only present in trace amounts. Usually, you wouldn’t investigate further; those traces are ‘not interesting’ for the current study.’
‘If you see something unexpected, then you have to be critical,’ says Hanefeld. ‘But don’t just say in advance that something can’t or shouldn’t be done.’ He cites the Diels–Alder reaction as an important example of this. ‘This reaction had clearly already been observed by others, but they were not open to it. So it was not Diels and Alder who discovered the reaction, but they were the first to describe it. That’s how the unexpected makes life more exciting.’
Perseverance is also important, says Hollmann. ‘No highly regarded journal wanted to publish our work at first, so we considered publishing it in a journal that publishes everything. This is partly due to the pressure to publish in China. Sometimes, they would rather publish more quickly in an unknown journal than conduct another experiment in order to publish in a more well-known one.’
Freedom
However, even in the Netherlands, the research culture is not entirely geared towards these kinds of unexpected discoveries. ‘In project proposals, you always have to indicate what the research is good for and what milestones you want to achieve,’ says Hanefeld. ‘But one of the Nobel Prize winners for Physics won the prize for a “Friday afternoon experiment”. Most of it is nonsense, but creativity and freedom are very important. Most project proposals are so rigid that there is no room for chance.’
Had Hollmann and Hanefeld written a proposal based on the contents of this paper, it would never have been approved because it was not anticipated that this enzyme would cause this reaction. Hollmann concludes: ‘Scientists need to be given more time to conduct “Friday afternoon experiments” and to try new things without having to immediately demonstrate their social impact.’
Wu, B. et al. (2026) Nat. Commun. DOI: 10.1038/s41467-026-68899-y












Nog geen opmerkingen