Leuven researchers have found a way to transform toluene into nitriles using electrochemistry, ammonia and… water! They report on this simple and potentially environmentally friendly procedure in ChemElectroChem.
As a chemist, if you discover a way to improve the synthesis of a widely produced substance, you should seize that opportunity with both hands. Sander Spittaels, Jef Vanhoof and Dirk De Vos from KU Leuven have found an electrochemical way to produce aromatic nitriles much more sustainably than was previously possible.
These compounds are used as solvents and as precursors for dyes, medicines and resins, and are produced on a massive scale. Any improvement is therefore welcome, provided it is also practically feasible.
That is certainly the case here: whereas the industrial process requires high temperatures and pressure, sometimes involving aggressive chemicals, the Leuven researchers have succeeded in using water, ammonia, and a simple catalyst (LiI) at room temperature and standard pressure.
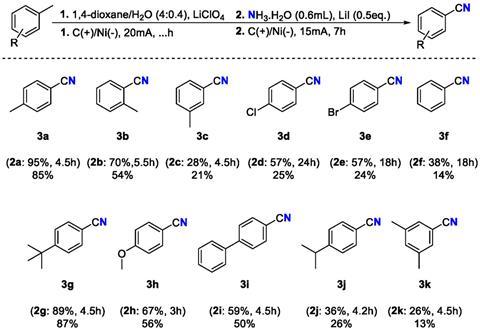
The researchers opted for the nitrilation of xylene and toluene derivatives because these are by-products of the cracker industry, with production reaching millions of tonnes per year. Having a sustainable synthesis route towards such substances naturally offers a great opportunity.
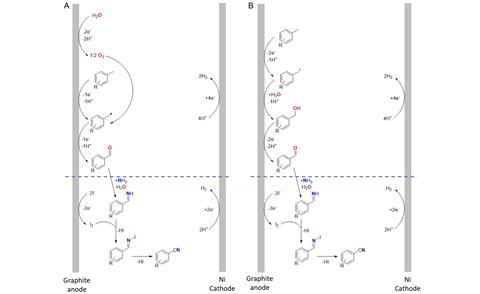
The Leuven process consists of two steps in a one-pot reaction. First, xylene is oxidised with water in dioxane under 20 mA for 4.5 hours to form an aldehyde. Then, after adding ammonia with LiI as a catalyst, the reaction continues for about 7 hours to form the nitrile. Over these two steps, a yield of 74% and a Faradaic efficiency (FE) of 34% were achieved. The reaction also proceeded successfully on a gram scale, offering hope for upscaling.
Spittaels, S., Vanhoof, J. and De Vos, D. (2025). ChemElectroChem. DOI: 10.1002/celc.202500267.












Nog geen opmerkingen