Polyurethane foam is found in mattresses, furniture and many other products, and it produces a waste stream that is difficult to recycle. However, a team from the University of Twente has now presented a sustainable method in Green Chemistry for converting this widely used material into reusable building blocks.
Ubiquitous in countless products, urethane foam almost always ends up in landfill or incinerators after use due to the lack of an efficient recycling process. Current processes require the use of toxic phosgene to reuse the building blocks. Consequently, large-scale recycling of urethane foam remains unattainable for the time being.
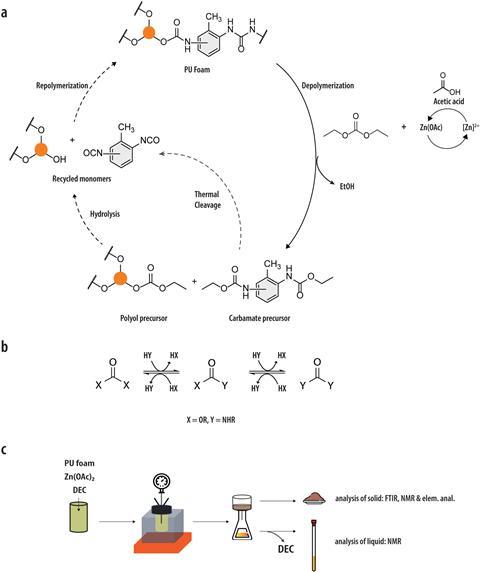
However, this may soon change, as researchers at the University of Twente have developed an environmentally friendly method of breaking down the foam into its original building blocks. And it doesn’t use dangerous chemicals. ‘With our chemical method, we depolymerise polyurethane foam into monomers, which can then be reused via a green process,’ says Jurriaan Huskens, a supramolecular chemist, professor at the University of Twente, and leader of the research project.
No phosgene
Current methods can already depolymerise polyurethane foam into one of its monomers, polyol. However, phosgene is needed to reuse the other monomer, diamine, in the formation of polyurethane. Unpaid professor and chemist Jean-Paul Lange, also from the University of Twente, decided to look for a better method and collaborated with Huskens.
Together with PhD student Ege Hosgor, the study’s first author, Huskens now demonstrates in Green Chemistry that polyurethane foam can be broken down harmlessly into both precursors using diethyl carbonate, which can then be repolymerised without phosgene. Huskens: ‘Under the right conditions, this allows ninety percent of both components to be recovered.’
The current method offers several green advantages and enables the foam to be synthesised in a circular way. ‘It avoids the production of toxic amines as an intermediate product and the release of chloride waste when using phosgene,’ says Lange. ‘In addition, the carbonate reagent can be made completely renewable from ethanol and carbon dioxide.’
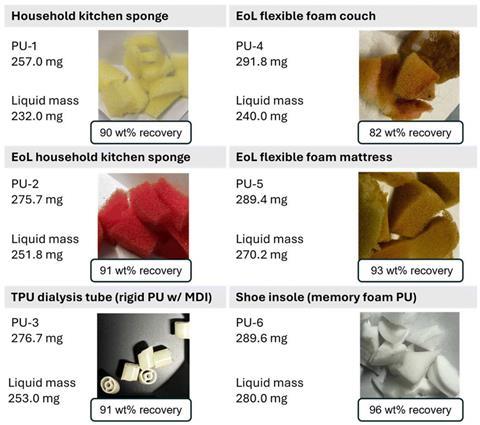
The researchers tested their reactions on different types of polyurethane foam, ranging from mattresses and furniture to sports soles and sponges. “We added small amounts of polyurethane foam directly to the diethyl carbonate,” says Huskens. “This quickly proved effective, provided we used a sufficiently high temperature and a catalyst — zinc acetate — to accelerate the reaction.” After letting all of these react together for a few hours, we ended up with a virtually liquid substance. It worked equally well for all the different types of polyurethane foam.”
However, that was when the real work for the researchers began. Huskens: ‘Analysing the product distribution of this complex mixture of substances was the biggest challenge. Initially, several oligomers remain in the mixture, and the amines that are not occupied by a carbonate occur in different isomers. We used NMR to study the distribution of monomers and oligomers, and the number of functional groups around the monomers. Although the chemistry itself went quite well quite quickly, the analysis still required a lot of external expertise.”
Potentially more expensive
As is often the case with breakthroughs in academia, a lot of work is needed to scale up the process for industrial use. ‘Our current process is quite complex and does not yet release the desired monomers, but only the precursors that can then be converted into the monomers,’ says Lange. ‘In addition, diethyl carbonate is more expensive than phosgene, so despite the reduced toxicity, I estimate that the process will be more costly than current methods.’
As a next step, the researchers therefore want to delve deeper into both the chemistry and the technical side of the process. Lange: ‘On the chemical side, we want to increase the yield of the reaction, see what unwanted by-products are created, and study how these affect the reaction adversely. On the technical side, we need to analyse the costs, determine the kinetics and thermodynamics of all the steps, and identify the most suitable equipment. We still have a lot to refine.’
Ege Hosgor, et al., Polyurethane depolymerisation by dialkyl carbonates: toward sustainable chemical recycling, Green Chemistry (2025), DOI: 10.1039/d5gc02533h













Nog geen opmerkingen