Amide synthesis has become much simpler with a new biocatalytic approach developed by researchers from Amsterdam. Their work was designated as a Very Important Paper in Angewandte Chemie.
Finding new, efficient and clean ways of synthesising amides is a major goal in chemistry. ’Until now, practical constraints have limited development, also in biocatalysis’, says Francesco Mutti, Associate Professor of Biocatalysis at the University of Amsterdam. ‘Over the past decade, researchers have managed to facilitate amide bond formation in aqueous environments, but this requires ATP-activated enzymes, which the industry is reluctant to use.’
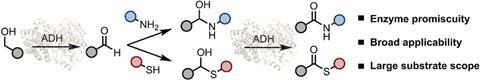
In contrast, Mutti, Matteo Damian and their colleagues have discovered a simple alternative that does not require ATP. ‘It’s very straightforward’, says PhD candidate Damian. ’Just mix an alcohol dehydrogenase enzyme in powder or liquid form with a cofactor, an alcohol and a second reagent to produce an amide through complete conversion.’ The team also uses air to supply oxygen. ‘Alcohols are cheap and abundant substrates, and we can play with both the nucleophile and the promiscuity of the enzyme’, adds Mutti. ‘There’s no reason for the industry to be anxious about using these enzymes.’
Lucky
Their publication in Angewandte Chemie International Edition about the synthesis of amides and thioesters with alcohol dehydrogenases (ADHs) was designated a Very Important Paper. ‘This work is especially important in the pharmaceutical industry’, Mutti elaborates, ‘as more than half of the top 200 drugs for retail sales contain amide bonds, which we created here.’ Damian adds, ’It’s not just the novelty of the work, but also its impact on the community. Our protocols are easy to implement.’
It all began with one of Mutti’s first PhD students, Jan Vilím, who wanted to convert alcohols to amines using a combination of enzymes. ’By serendipity, he discovered a new reaction involving an oxidase, which could convert alcohols into nitriles through a hemiaminal intermediate —a work also published in Angewandte Chemie in 2019’, Mutti recounts. Damian was inspired by this reaction. ‘I have a poster about his work on my office desk, and after doing some literature searches, I realised that I could do something similar with alcohol dehydrogenases through the same hemiaminal intermediate but forming amides this time.’ He was lucky, he says.
Apparently, the reactivity of ADHs is broader and is not constrained to ammonia as a nucleophile. Mutti: ‘We can introduce more complex amines and sulfur donors as substrates, and in the future it may work for some other nucleophiles as well. It’s a work in progress!’
Unconventional project
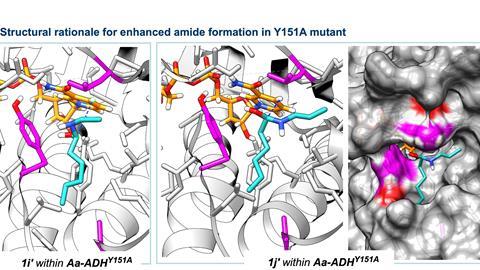
However, to achieve this, they had to take an unconventional approach. ’Everyone who works with enzymes knows that they don’t like high pH levels’, explains Damian. ‘But when I changed my perspective from biocatalysis to chemistry, we realised that high pH might work for the reaction we wanted, and subsequently our yields increased.’
The project was special in another way, too. ‘Matteo’s position was a bit of a “consolation position’’ for a larger grant application that was not funded’, says Mutti. ’Initially, there wasn’t enough funding to start the project, so instead the university provided funding for an internal PhD, which is very rare.’ This meant that there was a lot of freedom regarding both the project details and the PhD student. ‘The current system, in which proposals are evaluated by referees and committees, is generally considered to be the best possible situation. But sometimes, without scrutiny, the freedom allows unexpected results to emerge. The current peer-review system isn’t as flawless as we think. I doubt such a project would ever be approved. But if you don’t attempt crazy projects, significant discoveries will never surface.’
Damian adds, ‘Freedom is important, but at the same time, when I was a PhD student, despite my hard work, I didn’t have many positive results by the end of my first year. But Francesco still trusted me, which was really encouraging.’ Mutti shows a wide grin. ‘Matteo was doubting himself a bit back then. But failing is part of the process, and every PhD student goes through it. That’s why it’s crucial to show trust in your students, especially after the first year.’
Flow
For now, the scale is still relatively small at 500 mg. However, there are a few promising strategies that the team could implement to increase the yield and turnover numbers. For instance, immobilising the enzyme in a flow system could extend its lifetime, bypass the limited substrate loading and improve scalability. ‘Besides, we haven’t even performed any engineering yet to stabilise the enzyme further’, says Mutti in closing. ‘By pursuing state-of-the-art engineering methods to improve stability, the enzyme’s lifetime could be extended, allowing for greater productivity. Combining all these approaches would likely increase efficiency by orders of magnitude.’












Nog geen opmerkingen