CO2 can be used as a raw material in the production of thiocarbonate monomers. The resulting polymer turns out to be very suitable as a solid-state electrolyte in lithium-ion batteries, reports ChemSusChem.
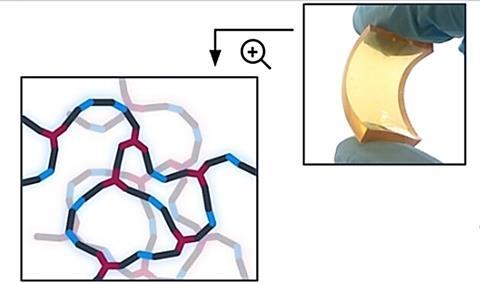
The use of CO2 in synthesis is not new. It can be seen, for example, in the relatively recent synthesis of poly(thio)carbonates. Thomas Habets, Christophe Detrembleur and colleagues from the University of Liège and the University of the Basque Country have now produced a variant that is liquid, has a lower melting point, is easy to synthesise and has interesting properties for an electrolyte in a lithium-ion battery.
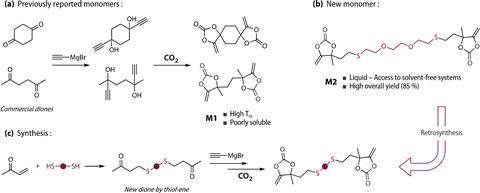
The synthesis starts with simple building blocks: two butenone molecules are coupled with the cheap compound 2,2’-(diethylenedioxy)ethanethiol, which consists of two thiol groups linked by a double ether. This makes the chain very flexible. You then react it with ethyne-magnesium bromide and couple the resulting molecule with CO2. This gives you the liquid monomer with a yield of 85% and it is scalable: the group went straight to making 60g of it to make polymers.
They then polymerised it into a cross-linked network and mixed it with the lithium salt LiTFSI to form an electrolyte. They compared its electronic properties with those of a previously synthesised polycarbonate. The use of (thio)ether compounds in the chain increased the conductivity by a factor of 2.5. The electrolyte is stable up to 4 V, making it suitable for lithium iron phosphate batteries.
The group will now further optimise the polymer for even better ionic conductivity, but they will also look at recycling: because of the sulphur bonds, the polymer can be decomposed and reshaped quite easily after heating.
Habets, T. et al. (2023) ChemSusChem e202300225, DOI: 10.1002/cssc.202300225












Nog geen opmerkingen