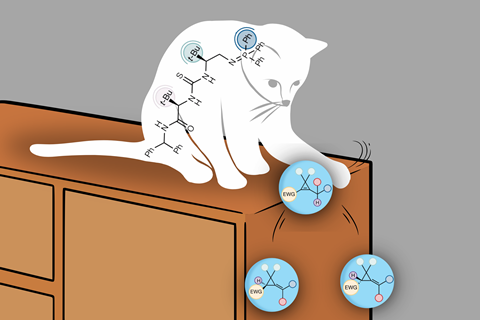
These essential building blocks are found in medicines and commonly used insecticides, but they are also difficult to synthesise: alkylidenecyclopropanes. In Nature, an international team presents a new method that makes producing these ring-shaped carbon structures much more efficient.
These small, triangular carbon rings are necessary for the production of certain medicines and insecticides. They occur as enantiomers — mirror-image versions of the same molecule — each with different properties. While one enantiomer is the active ingredient in a drug, the other has no effect or can even be harmful. The enantioselective synthesis of these carbon rings has long presented a challenge to organic chemists, and production has been inefficient until now.
However, researchers at the University of Oxford in England have recently developed a new catalyst that directs the reaction with high precision to the correct enantiomer. In collaboration with chemist Ken Yamazaki from Okayama University in Japan and the research group of chemist Trevor Hamlin from Vrije Universiteit Amsterdam (VU Amsterdam), they used computer models to study exactly how the reaction works.
PhD student Eveline Tiekink from VU Amsterdam investigated why the new method is so enantioselective using computational chemistry: ‘The synthesised catalyst has a unique three-dimensional structure that allows you to produce one of the isomers very selectively. Whereas previous reactions were fifty percent selective for one isomer or the other, there is now a ninety percent chance of producing the correct one.’
Quantum Chemistry
The catalyst, a chiral basic bifunctional iminophosphorane, directs the synthesis of the carbon rings with such high selectivity that waste products are minimised. Tiekink and Hamlin used quantum chemical simulations to understand why the reaction so often produces the correct enantiomer and not its mirror image.
‘The catalyst itself is stereospecific, which means that it has a unique structure that pushes the synthesis in one direction,’ says Tiekink. ‘Although this type of catalyst was designed in Oxford a few years ago, its impactful application in the synthesis of alkylidenecyclopropanes has now been realised.’
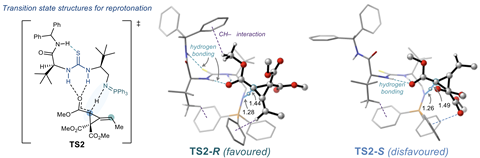
The researchers used computational chemistry software developed at VU Amsterdam. By running calculations on a supercomputer, Tiekink identified the reaction barriers involved in the synthesis. She employed density functional theory (DFT), a quantum mechanical method that calculates the energy of a molecular system and indicates the optimal position of the atoms. Tiekink: ‘It is also possible to run these simulations on your own computer, but in this case, each calculation would take weeks.’
Complex answer
Tiekink had to consider many factors in her calculations. For example, alkylidenecyclopropanes have many different enantiomers, resulting in a large number of possible reactions. On top of that, the molecules have a lot of conformational freedom, which increases the number of calculations further. ‘We had to express the large number of different conformations in the calculations using carefully chosen XYZ coordinates, which was a lot of work,’ says Tiekink. ‘Once we had done that, we could see which reaction steps were most important.’
However, summarising a clear answer to the question of why the catalyst produces such high selectivity remains the biggest challenge, according to Tiekink. The answer is not straightforward. ‘We looked at many different interactions, including π-π stacking, destabilising repulsion between electrons, hydrogen bonds, and so on. They all impact the result. As these interactions are not very strong, it is still possible for other enantiomers to be synthesised, resulting in ninety percent selectivity.’
Searching for more
The Oxford researchers, led by Darren Dixon, were able to use their catalyst directly for the efficient synthesis of the insecticide permethrin. Tiekink: ‘I am convinced that they will now continue to search for other possible reactions with this type of catalyst in Oxford.’
Hamlin, in turn, wants to use the insights from the DFT calculations to design the next generation of catalysts. Hamlin: ‘This theoretically based approach will guide our experiments, enabling us to create complex molecules with unprecedented precision. The potential to transform fields ranging from medicine to sustainable materials is enormous. We are only at the beginning.’
Golec, J. C. et al. (2025) Nature 645, DOI: 10.1038/s41586-025-09485-y











Nog geen opmerkingen