Using quantum chemical calculations, a Utrecht-Amsterdam team analyses the synthesis, reactivity and bond formation of nickel carbene complexes, reports Chemistry – A European Journal.
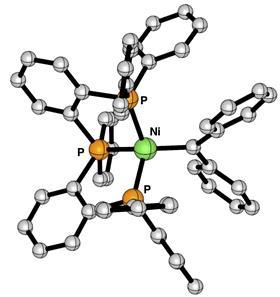
Catalysis with cheap transition metals is the dream of many chemists. Take nickel, for example. When it forms a double bond with, say, carbon (a carbene), it acts as an important intermediate in the synthesis of cyclopropane, for example. As always, it helps to know exactly how an intermediate looks, reacts and which ligand stabilises it. Researchers from Utrecht and Amsterdam joined forces to unravel the secrets of the nickel carbene complex.
‘For a long time it was thought that isolatable nickel carbene complexes were impossible’, says Marc-Etienne Moret, associate professor at Utrecht University. ‘But in the 2000s, Gregory Hillhouse’s group showed that with a few ligands – two in his case – you can create a situation where the nickel carbene is stable. His phosphine-bidentate ligand complexes were the only examples so far. So we were wondering: do you really need only two ligands, or can you use more?’
Four-ring
This question stems from earlier research by Moret’s group. ‘For some time now, we have been working on the chemistry of nickel carbenes and nickel cyclobutane, a four-ring made up of three carbon atoms and one nickel atom’, he says. ‘In fact, our long-term goal is to see if you can do olefin metathesis with nickel catalysts.’ This is currently done with rather expensive ruthenium-carbene complexes (such as Grubb’s catalyst), but there is no known efficient olefin metathesis with nickel, although it would obviously be much cheaper.
Postdoctoral researcher Pablo Pérez-García and PhD student María Sansores-Paredes are both working on nickel complexes in Moret’s group. Moret: ‘In our nickel cyclobutane there is a kind of olefin metathesis, but the carbene that is formed is still part of the ring. So we tried to create the same coordination environment, but without the carbene being part of the ligand. And it worked!’ The result was surprising, says Moret. ‘All sorts of questions immediately arose: why does it work with three phosphines and is it stable?’
‘Then his group knocked on our door’, says Pascal Vermeeren, associate professor of theoretical chemistry at VU University Amsterdam. ‘At the VU we do a lot of quantum chemistry on compounds and structures, including organometallic complexes. What I really liked about this project is that María came to our group for a while to learn the methods to look at the binding mechanism in detail. We can do it ourselves, but I think we should teach the new generation of scientists about all aspects of chemistry.’
Phenyl wings
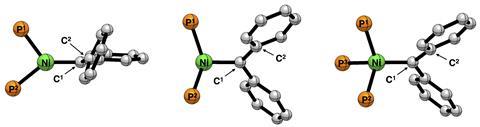
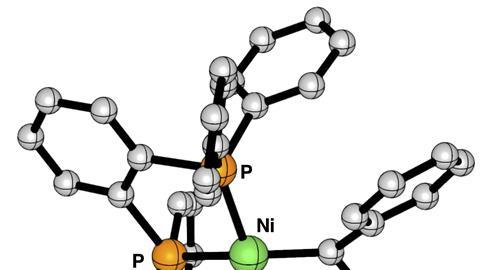
Sansores-Paredes first studied the Utrecht complexes and then compared them with those of Hillhouse. ‘Both nickel carbene complexes are stable, but in the Utrecht variants we saw that the interaction is weaker than in the bidentate ligands’, explains Vermeeren. ‘This seems to be mainly because the new system has large “phenyl wings” that provide a steric repulsive interaction with the incoming carbene.’
Although the bond is somewhat weaker and should therefore react slightly faster than the Hillhouse variants, the molecule is generally not as reactive. ‘It is precisely because it is so protected by these phenyl wings that our reactions have only been successful with small molecules such as CO’, says Moret. ‘The hypothesis is that the nickel centre is no longer really accessible, so our next step is to make a complex with less steric bulk around it.’
So, using quantum chemical calculations, the researchers first looked at the difference in the interaction. ‘It comes down to an orbital mismatch when there are three phosphine ligands coordinating to nickel, and that makes the interaction less stable’, says Vermeeren.
Dream reaction
Next, they wondered about the effect of this third phosphine-nickel compound on the complex. ‘In my opinion, this is where the beauty of theoretical chemistry becomes apparent’, says Vermeeren. ‘With computer models, you can look at things that are not possible synthetically, but which give you insight nonetheless.’ To do this, they started with a simple model of the Hillhouse complex: nickel with two phosphines and a carbene, without the rest of the ligands. ‘Then you gradually change the model to the Utrecht nickel-carbene complex. What do you find? The third phosphine bond pulls the electron density away from the nickel atom towards the ligand. As a result, you have less repulsive interaction along the bond axis with the carbene.’
‘The dream reaction is to do olefin metathesis with nickel’, says Moret. ‘This complex does not do that, but we have learned what the possibilities are.’ In the short term, he and his group plan to modify the complexes until they have a more reactive carbene. In the long term, this will hopefully lead to olefins. ‘But the most interesting part of this kind of work is discovering reactions that we don’t expect. That is ultimately the reason for making such reactive compounds.’
Pérez-García, P.M., Sansores-Paredes, M.L.G. et al., Synthesis, reactivity and bonding analysis of a tetracoordinated nickel carbene, Chem. Eur. J. (2024), doi: 10.1002/chem.202403211












Nog geen opmerkingen