Viral-vector-based gene therapies are finally a reality. The FDA has approved twelve gene therapies, with over 25 viral-vector therapeutics in late-stage development and nearly 150 in Phase II trials. Adeno-associated virus (AAV) has emerged as the primary vector for therapeutic gene delivery.
Despite rapid progress, significant challenges still exist in viral vector production. One such challenge is effective separation of full and empty capsids. Optimization of this step is challenging and critical to achieving good yields.
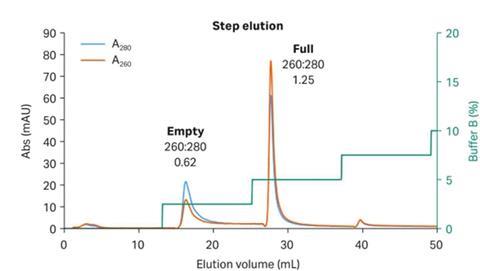
UV photometers have been used for decades to detect protein in post-column elution streams to automate product pooling and maximize yields. First-generation UV sensors utilized mercury lamps with broad emission spectrums, relying on narrow bandwidth optical filters to select wavelengths of interest (typically 280 nm for proteins). Capsids are essentially protein shells, so the 280 nm wavelength works well for capsid detection. Unfortunately, with a simple A280 measurement, there is no way to differentiate between empty and full capsids. DNA has an absorption peak at 260 nm, so while A280 measurement will represent total capsids (both full and empty), the A260 measurement will be proportional only to full capsids. As illustrated in Figure 1, the ratio of A260 / A280 provides a simple means of determining if your elution peaks are primarily empty or full capsids.
It is possible to utilize legacy UV sensor technology for this measurement, but traditional mercury lamps present challenges. Mercury vapor lamps have limited output at 260 nm, the absorption peak for nucleic acid (see Figure 2). Mercury lamps have a strong emission line at 254 nm which can be used to monitor nucleic acid concentration (slightly off the 260 nm peak), but the A254 results will differ slightly from the A260 measurement (typically only an issue when trying to align values during scale-up or process transfer).
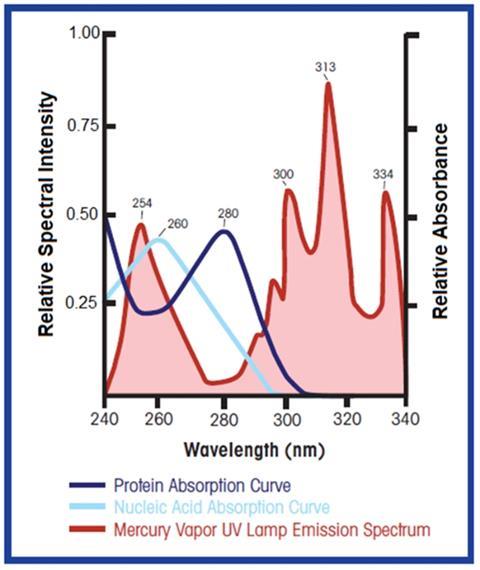
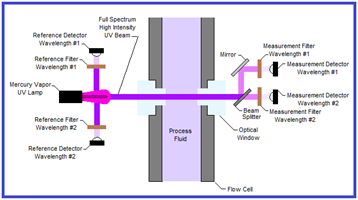
A second challenge with mercury vapor UV lamps is that high-intensity UV light has the potential to damage proteins and nucleic acids. UV light breaks down certain chemical bonds in RNA, DNA and proteins. As shown in Figure 2, the highest intensity output for mercury vapor lamps is outside the wavelengths of interest for monitoring proteins and nucleic acids. Legacy dual-channel UV sensors (see Figure 3) expose the process to full-spectrum, high-intensity UV light with narrow bandwidth filters on the detector side to block UV light outside the wavelength(s) of interest. Only a small percentage of the UV light that passes through the process is utilized in the measurement. This is primarily a concern in low-flow situations which subject the process to longer exposure times.
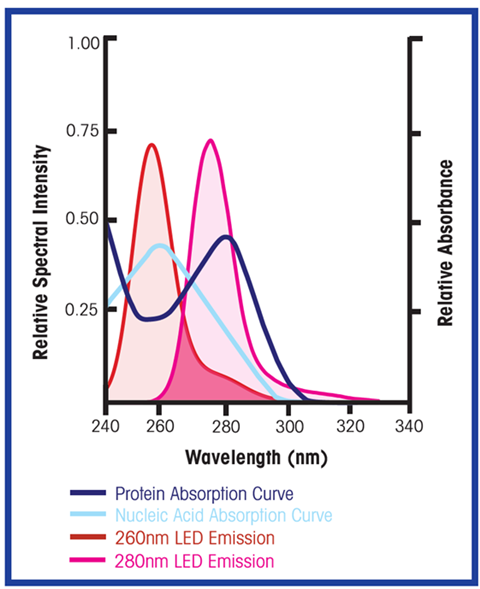
UV LEDs are now widely available and have started to replace mercury lamps. Their compact size, lower power consumption, longer lifespan and instant on/off capability make them ideal for inline spectrometry. Narrow-bandwidth LEDs centered at or near the wavelength of interest address the major challenges of legacy UV filter photometers. Proper LED selection provides strong output at the wavelengths of interest, ensuring excellent signal strength while limiting process exposure to extraneous UV emissions.
Viral vector production presents new challenges at every process step, including effective empty/full capsid separation. The industry has rapidly developed a new tool kit of enhanced resins, new matrices and new methods to optimize these processes. UV-LED based in-line photometers puts another tool in the hands of process scientists working to address these challenges.



