Steric repulsion is important to understand the bond formation process in molecules and your substituents might play a bigger role than you’d expect, an Amsterdam group reports in Chemical Science.
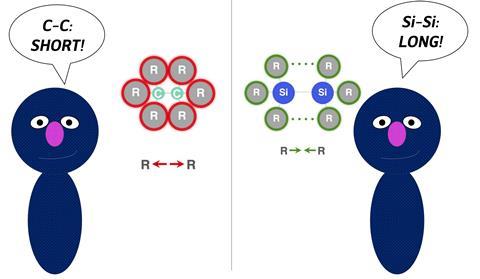
There are very little chemical bonds more thoroughly researched than the carbon-carbon (C–C) bond. That’s why it should come as no surprise that some binding concepts are intuitively understood. Take for example the size effect of substituents on the C–C bond. The bigger the substituents, the less stable the bond is. But to a certain extent, the reverse effect is true for Si–Si bonds: bigger substituents result in a stronger bond between the silicon atoms. Why is that? This question has been explored by Daniela Rodrigues Silva, Eva Blokker, Matthias Bickelhaupt and colleagues from the Free University Amsterdam in a collaboration with Nouryon.
‘The results may not seem surprising at first glance because this concept is very intuitive, but it is still often overlooked’, says Daniela Rodrigues Silva, who is a postdoc in the Amsterdam TheoCheM group. When talking about chemical bonds, researchers tend to explain them just in terms of the strength of the electron-pair bond being formed and do not see the effect of steric repulsion, she explains. ‘To understand the effect of the substituent size, it is important to first look into the intrinsic strength of group-14 element-element bonds. The Si–Si bond is weaker than the C–C bond, because of the increase in effective atom size and thus increase in steric repulsion.’ This effect can also be seen in carbon-halogen bonds, as Eva Blokker showed earlier. ‘This trend holds for other homodiatomic bonds as well.’
By doing DFT calculations and quantum chemical simulations, the group wanted to get at the bottom of this concept. They modelled R3C–CR3 and R3Si–SiR3, with R3 being H3 (small), Me3, Me2Ph, MePh2, Ph3 or t-Bu3 (large). Rodrigues Silva: ‘Our main conclusion was twofold: firstly, steric repulsion is very important to understand the bond formation process, and secondly, the same substituents can have opposite effects depending on the central atom it is attached to.’
Steric attraction
They exemplified this in the C–C and Si–Si bonds. ‘Groups around carbon are closer together and therefore more sterically congested’, Rodrigues Silva explains. ‘Silicon, however, is larger and has longer bonds, so the substituents feel each other much less, thus steric repulsion turns into steric attraction. As a result, the Si-Si bond gets stronger. This effect is further reduced when you go down the periodic table, to the point the bond becomes insensitive to the substituent size. This gives us knowledge to tune the bond strength at will.’
The fact that the Si-Si bond becomes stronger has to do with ‘steric attraction’ or ‘dispersion’. ‘Steric attraction is a less common term than dispersion, but it highlights the size effect’, says the postdoc. ‘This so-called steric attraction consists of stabilizing van der Waals interactions that take over and overcome the steric repulsion when the substituents are physically further away from each other.’
The researchers also found that the C–Si bond displays behaviour that is in between the C–C bonds and Si–Si bonds. Bigger substituents on C–C causes a weaker bond; on Si-Si it first causes stronger bonds until R=Ph3, then, for t-Bu, the bond gets weaker; for C–Si, bigger substituents cause weaker bonds, but not to the same extent as in C–C.
‘Our ultimate goal is to understand why things are happening’, Rodrigues Silva concludes. ‘Then we want to use this knowledge and link it to specific applications. We apply novel bonding models also in our collaboration with Nouryon, although for completely different types of systems.’
Rodrigues Silva, D. et al. (2024) Chem. Sci., DOI: 10.1039/D3SC06215E
Radial nodes
For the diehards: there’s a little more to the story. ‘When we talk about covalent bonds, we of course think about electron-pair bond formation’, says Rodrigues Silva. ‘When you go down the periodic table, the element-element bond becomes weaker due to steric repulsion between the bigger atoms, even though the electron-pair bonding orbital interactions get stronger. Why does the electron-pair bond become more stabilizing? Well, one of the reasons is the radial node. The sigma bonds arise from the bond overlap between pz-type orbitals. The more overlap, the stronger the electron-pair bond. In the case of the C—C bonds, the pσ-orbital already extends into each other’s angular nodal surface before the maxima of their lobes coincide, which causes less overlap and thus less stabilisation. This is different for the Si–Si bond because, due to the radial node in the Si 3pz AO’s, the maximum in the orbital’s lobe is pushed in the direction of the other atom, which causes the p-orbitals to have more overlap.’ To quote the paper: ‘(…) the radial node of the Si 3pz orbital (nonexistent in C 2pz) pushes the region of maximum amplitude of the 3pz lobe further away from the Si nucleus. This circumstance delays the out-of-phase overlap with the backside lobe of the 3pz [Atomic Orbital] of the other Si atom, resulting in a larger maximum [pzpz] overlap and, therefore, a larger Sbond [SOMO–SOMO bond overlap] for the Si–Si bond than for the C–C bond.’












Nog geen opmerkingen