Using dichloroethane and Reed’s benzenium ion as a catalyst, alkyl-substituted silicon can be very selectively modified, German researchers show in Nature.
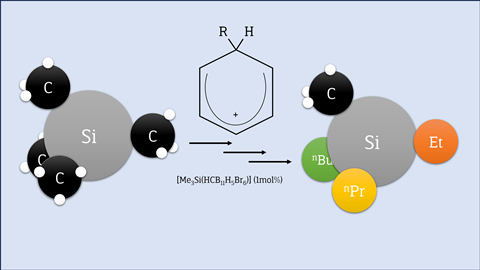
In organic chemistry, silicon is mainly found as a protective group for oxygen atoms, for example in the form of trimethylsilyl, because of its stability. But using silicon in a molecular structure and making Si–C bonds (silanes) has been quite difficult until now. Silicon with four alkyl groups is therefore currently considered a dead end. However, Tao He, Hendrik Klare and Martin Oestreich from the Technical University of Berlin have developed a relatively simple method for making and manipulating those dead-end silanes.
They use Reed’s catalyst, which consists of a benzenium ion and a boron bromide ion: [C6H6⋅H]+[CHB11H5Br6]–. They demonstrated the effect of this combination in Angewandte Chemie in 2018, but now they have shown a range of possibilities and the possible catalytic reaction mechanism. If you dissolve a tetramethylsilane into benzene with this catalyst and, say, 1,2-dichloroethane, you selectively replace a methyl group with a chlorine atom. You can then easily replace that chlorine atom in the next step with, say, a Grignard reaction, giving you a silane with three methyls and an alkyl group of your choice. You can do the same with disilanes (e.g. Me3-Si–Si-Me3). To prove their method, they also made a silicon-containing precursor of a drug.
He, T. et al. (2023) Nature, DOI: 10.1038/s41586-023-06646-9












Nog geen opmerkingen