An international team has developed a new method for making scalable photoanodes using inexpensive materials for solar hydrogen production, reports ChemSusChem.
The production of hydrogen as a renewable fuel is an important part of the energy transition. When you use renewable energy to produce hydrogen by water splitting, it’s called ‘green hydrogen’, but a specific subset of this is called ‘gold hydrogen’: using sunlight in photoelectrochemical water splitting to produce hydrogen and oxygen. Photoelectrodes that can do this have already been made, but their efficiency drops dramatically when scaled up. That’s why Pramod Kunturu, Marek Lavorenti and colleagues from DIFFER, TU Eindhoven and Toyota Motor Europe have developed a scalable method of fabricating the photoanode that retains 90% of its efficiency when scaled up.
To split water you need two electrodes, an anode and a cathode. Hydrogen is produced at the cathode, while oxygen is produced at the anode. Pramod Kunturu, a postdoctoral researcher at DIFFER, stresses that ‘we have not created a new photoanode material for oxygen production. It is a known semiconductor material that is already used for the oxygen evolution reaction in water splitting. We looked at the production method and what we could do to improve the performance.’
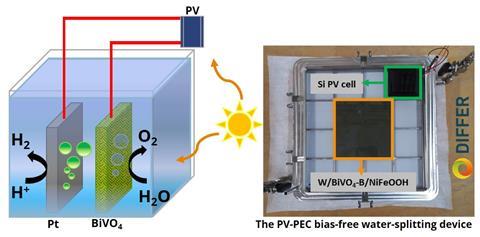
The group focused on BiVO4. ‘It is photo-active, which means that it generates a voltage when it is exposed to sunlight’, says Kunturu. This means you don’t need an external power source to drive the reactions. ‘However, we also wanted to improve the performance of BiVO4, so we doped BiVO4 with boron and added a NiFeOOH catalyst, which are cheap and abundant like the BiVO4 itself.’
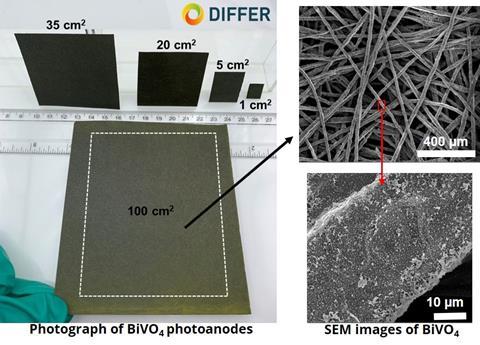
The researchers used a method they call SILAR: successive-ionic-layer-adsorption-and-reaction. Kunturu explains: ‘It’s a solution method where you dip your device into one solution and then into another to make BiVO4. The process is sensitive to the duration of the dip and the way you dip the device. It was quite a challenge; on a lab scale, say 1 cm2, you work with simple equipment, but making a 100 cm2 plate is quite a different task! But in the end we managed to get a uniform coating.’
However, 100 cm2 isn’t the upper limit. Kunturu confirms that they can produce their photoanode on a metre scale. ‘This is the first time we have shown that it is possible to use a porous material as a substrate and achieve this performance at this larger scale. But we’re just getting started.’ The same goes for the reported efficiency. ‘For photoelectrochemical water splitting, we have an industry target of 10% solar-to-hydrogen efficiency’, says Kunturu. ‘Although the 2.2% efficiency we achieved may not seem like much at this point, the current work on larger scale [100 cm2, red] PEC devices is very promising and may eventually represent a major leap in PEC technology for solar-to-hydrogen production.’
Kunturu, P.P. et al. (2023) ChemSusChem e202300969, DOI: 10.1002/cssc.202300969












Nog geen opmerkingen