Raising the temperature of electrocatalytic NH3 synthesis with H-permeable Ni electrodes makes production higher, more stable and more efficient, reports ChemSusChem.
Ammonia (NH3) is the basis for nitrogen-based fertilisers, of which 180 million tonnes are produced annually. Efficient synthesis is therefore essential. While the Haber-Bosch process is currently the most widely used, researchers are now looking for alternative methods without fossil fuels. Electrocatalytic reduction of nitrogen gas is one such alternative, but so far it has not been efficient enough because, among other things, the catalyst is fouled by NOx. Davide Ripepi, Herman Schreuders and Fokko Mulder from Delft University of Technology have developed an improved method that is much more efficient.
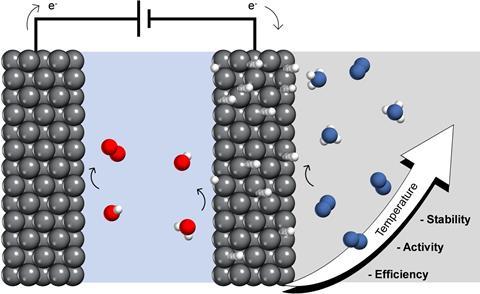
Previous research had already shown how to prevent catalyst fouling with a hydrogen-permeable nickel electrode. The system is designed to have two separate parts, with the water oxidation reaction as the electron source in one part. At the boundary of the other part, the water releases a hydrogen atom which permeates through the electrode to the part where only nitrogen (and no oxygen) is present and adsorbs on the electrode. There, the hydrogen can react with the nitrogen to form ammonia.
At fairly standard temperatures and pressures, this method works, but the performance is still far below a usable level. So Mulder and his colleagues played with the temperature. They saw promising results when they raised the temperature to 120°C. Indeed, this increased the rate of NH3 synthesis and also ensured a stable catalytic cycle.
The authors speculate that this is because nitrogen adsorption and ammonia desorption are both improved, so that the catalyst is constantly covered with nitrogen atoms. In addition, the higher temperature also allows for more efficient electrochemical hydrogen supply, which increased the Faraday efficiency by a factor of 37 and even reduced the electrical energy required. They expect further modifications to significantly improve ammonia yields.
Ripepi, D., Schreuders, H. & Mulder, F.M. (2023) ChemSusChem e202300460, DOI: 10.1002/cssc.202300460












Nog geen opmerkingen