When cyclometallation ligands are used around iron(III), it has an excited state that lasts a thousand times longer. This appears to be a first step towards photocatalysis with iron, say German researchers in Nature Chemistry.
Photocatalysis is one of the emerging techniques for producing solar fuels. So far, researchers have mainly worked with the relatively efficient complex [Ru(bpy)3]2+, but ruthenium is a rather rare and expensive transition metal. It would be much better to use metals from the row above, such as iron, which are much more abundant on Earth.
‘You need molecules that can absorb light, separate charges and extract electrons from the environment’, says Sylvestre Bonnet, professor of bioinorganic chemistry at Leiden University. German researchers from the University of Paderborn and elsewhere seem to have found such a complex. They have synthesised an iron complex with a relatively long excited state.
Such an excited state is necessary for photocatalytic complexes. ‘When a molecule captures a photon, it goes into an excited state,’ explains Bonnet, who was not involved in the research. ‘This state has a certain lifetime. For the molecular world, [Ru(bpy)3]2+ has a state that is relatively long, at one microsecond. Iron’s excited state usually lasts a few picoseconds, but in this paper it reaches 4.6 nanoseconds.’
Blue
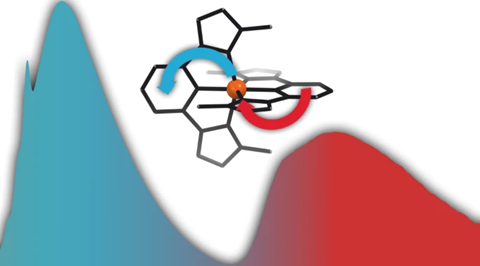
The complex consists of an iron(III) ion surrounded by two identical ligands that wrap around the iron ion. The ligand consists of a benzene ring with an imidazole at positions 1 and 3. Carbon 2 of the benzene and the carbons between the nitrogen atoms on the imidazole molecules act as carbenes, carbon atoms with a lone pair of electrons that the ligand donates to the iron ion. The result is a blue iron complex that is stable in water and air.
Normally, iron has several excited states, so the molecule can return to the ground state in stages. ‘This leads to a much shorter lifetime’, says Bonnet. ‘The researchers wanted to give these intermediate states a higher energy so that it doesn’t fall back step by step, but at once.’ They did this with carbenes, which are very electron-rich and thus increase the level of the excited states.
The interaction between the ligands and the metal ion causes two excited states to follow each other: first a ligand-to-metal charge transfer (LMCT) state of 240 ps, then a metal-to-ligand charge transfer (MLCT) state of 4.6 ns, which, according to the authors, is ‘the longest charge transfer excited state lifetime of iron complexes reported so far’. What has also not been shown before is the double emission (Janus emission) that occurs, resulting in a bright blue colour.
Supramolecular
The next step would be to find a suitable catalyst that can be driven by this complex for catalytic reactions. ‘The 4.6 nanoseconds is longer than the picoseconds, but it is still short compared to the microseconds of ruthenium’ , Bonnet says. ‘ But maybe we don’t need to go that far. Between ten and a hundred nanoseconds, photocatalysis could already be achieved, especially if you fix the components in a supramolecular environment so that the distance between them is short and electron transfer is easier.’ At the very least, the first step has been taken.
Steube, J. et al. (2023) Nat. Chem., DOI: 10.1038/s41557-023-01137-w












Nog geen opmerkingen