Silicon, germanium and tin versions of carbenes make good hydrogen activators for hydrogenation reactions, quantum calculations show. They could take over the role of transition metal catalysts, a team from Amsterdam shows in the European Journal of Organic Chemistry.
Hydrogenation reactions, in which activated hydrogen reacts with another molecule, are usually carried out with catalysts based on (expensive) transition metals. In their search for alternatives, Eveline Tiekink, Siebe Lekanne Deprez, Pascal Vermeeren and Trevor Hamlin from the TheoCheM group at VU University Amsterdam landed on group 14 elements.
Back in 2021, the team had already studied these so-called metallylenes when it was discovered that they could activate hydrogen and other small molecules. Tiekink and her colleagues have now found out whether it is possible to use rational design to develop metallylene catalysts that can carry out hydrogenation reactions. And they can.
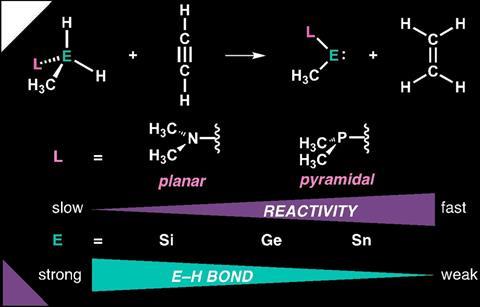
Using computational chemistry, they mapped the reactivity and bond strength of the model metallylene H3C-EH2-L – where E stands for C, Si, Ge or Sn and L for the ligand NMe2 or PMe2 – in the hydrogenation reactions with C≡C, C=C, C=N and C=O.
The calculations showed that the lower you go in the periodic table (i.e. from C to Sn), the lower the reaction barrier and the more energy is released. If the ligand is varied, the opposite is true: from NMe2 to PMe2, the reaction barrier becomes higher and less energy is released. In turn, these two trends are exactly reversed when looking at hydrogen activation.
Together with the other analyses in the paper, this provides the basis for an ideal catalyst for a given substrate. For the bonds mentioned above, this was either H3C-Ge-PMe2 or H3C-Sn-PMe2. Indeed, PMe2 lowers the reaction barrier for hydrogen activation and a large group 14 atom does the same for the hydrogenation step. The authors’ practical advice for those wishing to use a metallylene for hydrogenation is to use a large central atom, such as tin or germanium, and to optimise a phosphorus ligand for hydrogen activation.
Tiekink, E.H. et al. (2024) EurJOC 27(14), DOI: 10.1002/ejoc.202400069












Nog geen opmerkingen