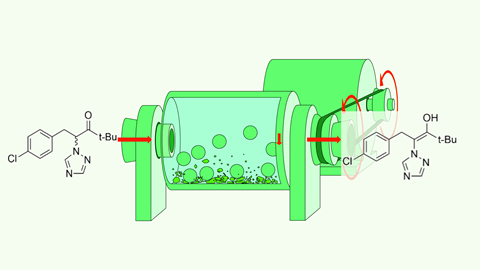
If you put a racemic mixture through a ball mill, one of the enantiopure forms will come out the other side within a few hours. The Franco-Belgian researchers claim to be the first to ‘deracemise’ with mechanochemistry in Chemistry – A European Journal.
A racemic mixture consists of a set of mirror-image isomers (enantiomers) in equal proportions. However, for many (biologically relevant) applications, only one of the two enantiomers is needed. The other form may even be harmful. Researchers are therefore looking for a simple way to separate these enantiomers. Chrystal Lopes, Tom Leyssens and colleagues from UC Louvain and Université Rouen de Normandie appear to be using brute force: by grinding a powdered form of a near-racemic mixture with small beads, they obtained an enantiomeric excess of up to 97%.
Specifically, they looked at the precursor of paclobutrazol: 1-(4-chlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pentan-3-one (see figure). Paclobutrazol is a plant growth inhibitor and is supplied as a racemic mixture, although the two enantiomers have different biological roles. Viedma ripening is the usual method for separating this type of enantiomer. In a nutshell, you dissolve your substance in crystalline form and crush it, forcing the crystals to grow into a single enantiomer due to the chiral crystal structure. The downside of this technique is that, in this example at least, it takes several days to get a separated mixture and it also consumes a lot of solvent.
However, Lopes and colleagues managed to do it a bit faster: it took only three hours to react a mixture with a small enantiomeric excess (10%) to an enantiomeric excess of 77-97% in a high-energy milling process. Furthermore, they only needed a catalytic amount of water and a little base to get the reaction going. Although they do not yet fully understand the underlying mechanisms, the step towards ‘greener deracemisation’ seems to have been taken, say the authors.
Lopes, C. et al. (2023) Chem. Eur. J. e202300585, DOI: 10.1002/chem.202300585












Nog geen opmerkingen