A ring-shaped molecule now makes it possible to control the self-assembly of nanoparticles from the outside, according to an Amsterdam-based group writing in Nature Chemistry.
The synthesis of crown ethers, or the self-assembly of small nanoparticles, involves using templates such as anions or cations around which molecules form. ‘These are always at the centre’, says Joost Reek, Professor of Homogeneous and Supramolecular Catalysis at the University of Amsterdam. ‘If you look at this type of self-assembly process, you see a small guest [the template, ed.] around which a structure is built. This is called an endo-template.’
Reek and his team have now reversed this process by using exo-templates to influence self-assembly from the outside. ‘This is something completely new. Instead of folding around something, we are showing that you can also fold from the outside in for the first time’, Reek explains. ‘This is not that relevant for small structures, but our nanostructures can be up to five nanometres in diameter. They are so large that there are no suitable endo-templates available for them.’
Test tube
Endo-templates have long been very functional for small structures. However, as researchers now want to build larger and more complex structures, the energy landscape is expanding. ‘The procedure then becomes something like this: mix the building blocks you’ve made and hope that the product forms thermodynamically rather than kinetically’, Reek continues. ‘With larger structures in self-assembly, you need new tools for pathway engineering — it no longer happens automatically.’
He gives the example of a living cell. ‘If you put all the fantastically organised components of such a cell individually in a test tube, they will never just form a cell by themselves. That requires external control.’ This is one of the big questions in chemistry and biology. In the future, Reek’s group intends to develop additional tools to facilitate this type of biomimetic self-assembly; however, this work constitutes a modest initial step in that direction.
Solar cells
The exo-template from this paper consists of pseudorotaxanes. Reek: ‘A rotaxane is generally an interlocked system consisting of a ring-shaped molecule and an elongated molecule that are attached to each other without a covalent bond. However, they are arranged in such a way that the ring cannot slide off. In pseudorotaxanes, a similar structure forms a ring around an elongated molecule; but in this case, the ring can slide on and off.’
Article continues below the image
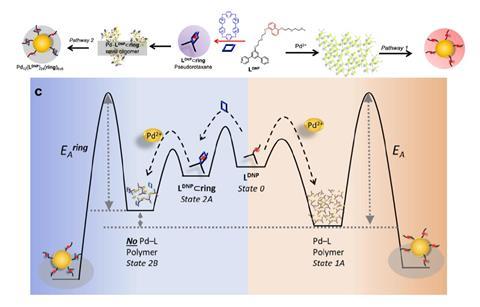
Surprisingly, these pseudorotaxanes originate from solar cell research. ‘The first author, Tessel Bouwens, who is now an assistant professor at TU Delft, was working on dye-sense-dye solar cells’, says Reek. ‘Two years ago, we published a paper on this in Nature Chemistry, in which Tessel used pseudorotaxanes in ‘dye-sense-dye’ solar cells.’ At one point, Reek suggested that she should broaden her horizons because she was so focused on that. Bouwens then decided to use the pseudorotaxanes to create nanostructures. Slowly but surely, the research project began to take shape.
The effect of the pseudorotaxanes — the exo-template — is the physical bending of the nanostructures and a change in the energy profile. ‘Without a template, you first get a long polymer, which turns into a flat polymer and then slowly forms the thermodynamic product,’ says Reek. The exo-template destabilises the intermediate state, resulting in a catalytic effect. ‘Essentially, it is an assembly catalyst. However, according to one of the reviewers, the focus should be on exo-templating. The point is that the intermediate state increases slightly in energy instead of the transition state peak lowering.’
Energy profile
As this approach is so new, a whole world of developments is still open to exo-templates. For example, you could make nanocages based on a mixture of building blocks, says Reek. ’If you mix L-methoxy and L-naphthalene in equal parts, you get a statistical distribution of the composition of the nanoparticles. Yet if you do the same in the presence of pseudorotaxanes, the distribution becomes much narrower.’
Therefore, if you first mix the molecules with the exo-template and then without it, you get a mixture of cages that are not statistically distributed. ‘They are then entropically higher in energy — essentially an out-of-equilibrium system that slowly returns to equilibrium. And that can lead to all kinds of interesting applications.’
In principle, you could also use different templates. ‘But that field is completely open. Our main focus is on applications in catalysis and light harvesting for generating solar fuels. We are now looking at mixing building blocks because exotemplating allows you to introduce much more structure in a controlled manner. But it still requires a lot of research!’
Bouwens, T. et al., 2025, Nat. Chem., DOI: 10.1038/s41557-025-01808-w












Nog geen opmerkingen