Groningen-based researchers designed an oscillating small molecule reaction that catalyses another reaction in the same system, Nature reports. This is the first time that such a complex synthetic system actually works.
Even though oscillatory reactions are very common in living cells, creating synthetic variants is an art in itself. In this type of reaction, such as the iodine clock reaction, chemical reactions alternate, creating a wave-like (oscillatory) pattern. This can be seen, for example, in colour changes. Most oscillatory reactions are based on inorganic reactions. These are not really practical because you have to work in very acidic conditions. Another option is enzymatic oscillation, but the disadvantage is that enzymes are not easily adaptable.
‘These are all oscillating systems that are not coupled to any other chemical reaction’, says Syuzanna Harutyunyan, professor of homogeneous catalysis at the University of Groningen. ‘What our work shows is a complete organic oscillating system that also has a catalytic function in another reaction. At one point we had thirteen compounds in one flask. This is not normally possible in organic chemistry, but my PhD student Matthijs ter Harmsel’s design made it work.’
Piperidine
Ter Harmsel applied to Harutyunyan’s lab about four years ago for a PhD project, and she asked him if he would be interested in a project involving oscillations. ‘I knew little about it’, Ter Harmsel says, ‘Just the videos on YouTube. But I was intrigued by the subject and immediately started working on a design.’ Within a few weeks, Ter Harmsel had a workable idea: at the centre is an autocatalytic process around which you build inhibitions and reactions. The system would become quite complex, so you have to make sure that everything else remains simple.
‘To make this work, you have to look for organic molecules that are good catalysts and can also undergo autocatalytic and inhibition reactions’, explains Harutyunyan. ‘Piperidine is known to be a good organic catalyst, so you can use it for a combination of the processes you need: an autocatalytic reaction where more piperidine is released, and another reaction where piperidine is consumed.’
‘I was looking for a relatively simple autocatalytic reaction involving piperidine, and the Fmoc deprotection came out on top’, Ter Harmsel continues. The Fmoc group (fluorenylmethoxycarbonyl) is a common protective group used to protect amines. This became the starting point for the reaction chain: first autocatalysed Fmoc-piperidine deprotection, then N-methylpiperidine-catalysed Fmoc-piperidine deprotection, rapid inhibition by acetylation, slow inhibition with phenyl acetate, and simultaneously a piperidine-catalysed chemical reaction between two reactants. The concentration of the reagents was monitored by in situ IR spectroscopy (see Figure 1).
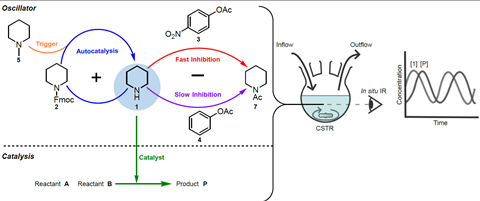
This way, you can see the concentration rise and fall, resulting in a single pulse. ‘It took us two years to get that one pulse’, says Harutyunyan. ‘At the time, we also talked to Albert Wong from the University of Twente, who has experience in modelling complex systems. For an oscillation, you obviously need a lot of pulses.’ At some point she got in touch with Wilhelm Huck, professor of physical organic chemistry at Radboud University. Huck has a lot of experience with enzymatic oscillations in flow systems. Harutyunyan: ‘In a batch, you only get one pulse. For oscillations, the system has to remain out of equilibrium, which you can achieve with flow; you then mimic many batch reactions.’
Time-dependent
So Ter Harmsel spent a week in the Huck laboratory where he learned the intricacies of flow systems. ‘Together with Oliver Maguire, I sometimes spent up to 12 hours in the lab, which gave me the technical know-how we lacked in Groningen’, says Ter Harmsel. ‘That way we didn’t have to reinvent the wheel. Instead, I was able to take that knowledge with me to build something similar in our own lab.’ Laughing: ‘I jokingly called our system the “Harmselator” during a meeting and the name stuck.’
With the oscillation in hand, the next question was what reaction it could be linked to. Harutyunyan: ‘Knoevenagel condensation seemed like a good option: in theory, the reagents should not interfere with each other, and in practice it turned out to be the right choice. It’s also a fast reaction.’ This is important because your oscillation peak is time-dependent. This means that the reagents only have a short period of time to react with each other.

When the project was well under way, Sofiyah Runikhina joined the team. ‘She was really focused on applications’, recalls Harutyunyan. ‘Her involvement was mainly in the catalysis part of the oscillator.’ Ter Harmsel: ‘The three of us started brainstorming on how to take the whole project one step further. We divided the ideas between us and started trying all sorts of things. At one point, things weren’t working so well and as we stood in front of the fume cupboard, Sofiyah said: “Nothing seems to be working. Can’t we just try to use what doesn’t seem to work?” That turned out to be a good idea: one reaction didn’t work and another did, and thus we had introduced selectivity into the system. Now a postdoctoral researcher, Runikhina is looking for other possibilities.
Tandem
Because these possibilities certainly exist, says Harutyunyan. ‘If you look at a cell, you see all sorts of reactions happening in parallel, in a very controlled way. I think controlling such complex systems is where we’re going in the future. Ideally, we would like to have different oscillators with different kinetics. We are already looking at this in our group: at the moment we are working on tandem oscillations: when the first one starts, the second one is turned on, which in turn stimulates the first one, and so on.’ Another practical application the team is considering is linking the oscillator to polymerisation. ‘You could then get different polymer chains depending on the kinetics of the oscillation. But at the moment I’m just speculating, mind you’, she says with a laugh.
It is getting more and more complex and modelling is becoming increasingly important. ‘There are so many parameters changing at the same time. That’s why you have to combine modelling with experimentation. It may be difficult, but I really believe that it is possible to create such a complex system’, Harutyunyan concludes.












Nog geen opmerkingen