Chiral molecules are nothing new. However, chiral molecules with their sole stereogenic centre on an oxygen atom are something completely different. British researchers are the first in the world to accomplish this.
Chirality, or ‘handedness’, is a fundamental concept in chemistry. The vast majority of the molecules in your body, for example, are chiral, meaning that they rotate polarised light either to the left or to the right, depending on their spatial structure. Usually the chiral centre is a carbon atom with four different substituents, but in some cases it’s trivalent phosphorus, sulphur or nitrogen. In these latter atoms, chirality can be switched by pyramidal inversion, just as the wind sometimes ‘inverts’ an unfortunate umbrella. But researchers at the University of Oxford were able to synthesise a room-temperature stable molecule with its only chiral (also called stereogenic) centre on an oxygen atom – a world first – which they published in Nature.
‘Using their quantum calculations, we were pointed in the right direction’
Martin Smith
‘We didn’t actually set out to design this molecule’, says Martin Smith. He and his colleague Jonathan Burton are both professors of organic chemistry at the University of Oxford. ‘But it came together rather organically.’ Although the original idea had something to do with catalysis, the team pursued the stereogenic oxygen idea thanks to some very proactive PhD students.
Tricycloalkyl cage
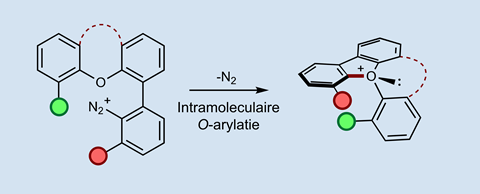
The molecule is based on the triaryloxonium ion, a positively charged oxygen atom to which three benzene molecules are directly attached. Smith says: ‘Jon and his group already have a significant history in the synthesis of oxonium ions.’ The idea came from a structure they had made previously, although it wasn’t the focus of that study. ‘We embedded an oxonium ion in a tricycloalkyl cage, and the presence of other stereocentres made it stereogenic’, Burton adds. ‘Together with Martin’s earlier work on ammonium ions, and driven by the enthusiasm of our students, we decided to investigate this further.’
‘The helix structure keeps the oxygen atom from inverting too quickly’
Jonathan Burton
But there are so many possible oxonium ions, where do you start? ‘Fortunately, Robert Paton and his Postdoc were able to do quantum calculations that pointed us in the right direction’, says Smith. After a short synthesis with reasonable yields, they ended up with a helical molecule with an oxonium ion in the middle. ‘It was surprising to see that oxonium ions are so accessible and remarkably stable. They’re also relatively easy to make in the hands of a good graduate student.’
Cool
Normally, pyramidal oxonium ions – similar to ammonia – perform billions of inversions per second. ‘That is why the structure had to be helical’, explains Burton. ‘It prevents the molecule from inverting too quickly.’ Because of the helical structure, the half-life of racemisation has now been increased to about 18 days, which is enormously slower.
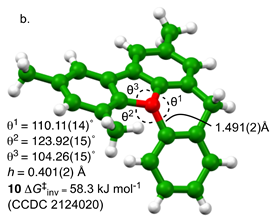
‘I think it’s a really cool molecule,’ says Burton with a laugh. ‘And I hope others will think so too. That’s the beauty of fundamental science, isn’t it? Seeing what’s possible. I think this work is a nice addition to the field of stereochemistry.’ Although it’s mostly fundamental, there may be some applications. ‘We’ve at least shown that there is a basic scaffold for slowing down oxonium inversion’, says Smith. ‘There are lots of ways to change the barrier, to play with the chemical make-up. And triaryloxonium ions are also useful to chemoselectively make benzynes, which are hyperreactive intermediates, under super mild conditions. But the biggest output is that there are now three fully trained PhDs who will ultimately make the world a better place.’
Comments
‘It is an extremely elegant piece of work’, says Jan van Maarseveen, Professor of Synthetic Organic Chemistry at the University of Amsterdam. ‘I am particularly pleased with the way they have approached the synthesis. Instead of making a molecule and then using computational methods to confirm the structure, they did it the other way round: first they calculated what the molecule should look like, and then they synthesised it accordingly.’
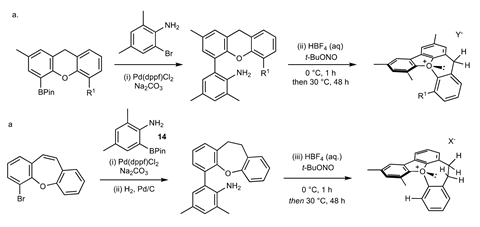
Although he is full of praise for the work, Van Maarseveen does have one or two comments. ‘The researchers had to separate the enantiomers that were present in equal amounts. What would improve this synthesis is to enantioselectively synthesise the oxonium ion from the start. But I’m sure they’re already working on it.’ There’s one thing that disappoints Van Maarseveen: ‘They didn’t report the optical rotation! For such an interesting chiral oxygen atom, I would have expected it.’
Bottomline, Van Maarseveen is excited about the potential applications. ‘It must be useful somehow for asymmetric organocatalysis. I think there will be many interesting use cases.’ The biggest takeaway from this work? ‘Good design pays off.’
Smith, O. et al. (2023) Nature 615, doi.org/10.1038/s41586-023-05719-z




Nog geen opmerkingen