A home-made photochemistry robot could significantly advance the field of organic (photo)chemistry with rapid yield optimisation, easy scale-up and reliable reproducibility studies, an Amsterdam-based group reports in Science.
Photochemistry is a promising but still relatively young field that aims to use light to catalyse chemical reactions. As a result, photochemists still have many problems to solve. Combining it with flow chemistry, another relative newcomer, has already made great strides. But there are still difficulties: optimisation, reproducibility and scale-up are three major challenges. With RoboChem, Aidan Slattery, Timothy Noël and colleagues at the University of Amsterdam are killing these three birds with one stone.
Spot on
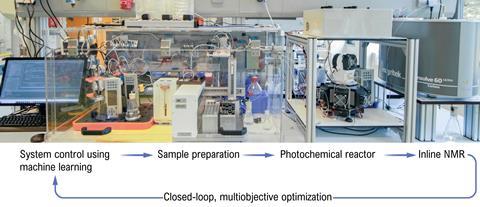
RoboChem is a group-built photoflowchemistry synthesis robot that optimises and scales up chemical reactions completely independently. The platform proves itself in the paper by improving almost every specified reaction, not only in terms of yield, but especially in terms of space-time yield, the amount of grams per litre per hour that the robot synthesises. Depending on the reaction, RoboChem can easily produce more than a kilogram per day.

‘This work eclipses everything else’, says an enthusiastic Noel, professor of flow chemistry. ‘We have written a behind-the-scenes blog that shows the work that has gone into it, but also the excitement of texting each other the latest results. We could have published this a year ago, but we scaled it up, isolated it, checked each molecule several times to really make sure everything was reproducible. It was always spot on.’
Differences
The Noël group tested five photochemical reactions: C-H alkylation and C-H trifluoromethylthiolation via photocatalytic HAT; aryltrifluoromethylation and oxytrifluoromethylation via photocatalytic SET; and C(sp2)-C(sp3) cross-electrophile coupling. It became apparent that RoboChem’s artificial intelligence sometimes differed significantly from the literature in terms of reaction conditions.
‘I kept telling my students: “We have to calm down, soon they will think we are faking it!”’
For example, when comparing the trifluoromethylthiolation of ambroxide with that of sclareolide (two very similar substances), the robot used much more intense light in the latter reaction than in the former. In retrospect, the authors write, this choice was rational: Ambroxide can undergo a different reaction that is not possible with sclareolide. Less intense light and less catalyst therefore resulted in a higher yield with ambroxide.
‘It was quite striking’, says Noël. ‘With certain substrates, you would normally go for maximum light intensity, up to 180 watts. But RoboChem chose to use only 2 watts for one substrate. If we had done it ourselves, we would probably have blown it up and thought the substrate was unstable. We could not have predicted such a big difference beforehand with similar reactions going up to 140 watts.’
Continue below the images
Python
The optimisation was surprisingly good, says Noël. ‘Normally you optimise one substrate and then copy those conditions to other substrates. But this machine treated each substrate almost like personalised medicine: it looked at functional groups, electronic properties, steric hindrance.’ In doing so, RoboChem almost always achieved at least the literature yields, and usually improved on them. ‘On the advice of one of the referees, we had to do an even more complex reaction, cross-electrophile coupling. But the more complex the problem, the more benefit we saw from optimisation. I kept telling my students: “We have to calm down, soon they will think we are faking it!”’
‘With this system, we hope everyone will try flow chemistry’
The group did not expect such good results. ‘I already told my students: this is probably the best thing we ever did’, Noël continues. ‘And that was also because of the collaboration: chemical engineers, chemists, analysts and so on, everyone had their role. The “chemistry” between the people was just so good. Of course, we had to learn a lot ourselves and set everything up ourselves; for example, students learned to code in Python from YouTube videos, I sent many of them to seminars and workshops; I didn’t have the knowledge, so someone had to pick it up’, he says laughing.
Data
The platform is not extremely expensive either, and all the code is available online on GitHub. It does take time and knowledge to put it together, but: ‘According to the feedback we have received, this is one of the most accessible platforms that is feasible for many,’ says Noël. ‘The most expensive thing remains the analytical equipment, but the rest is not too bad. The hardest part is to bring all the various skills together.’ The group is also trying to 3D print the pumps and make the whole thing more accessible. ‘Currently, I almost always have to explain what flow chemistry is, but with this system we hope everyone will try flow chemistry.’
One of the best things about RoboChem, according to Noël, is the data it generates. ‘Have a look at the Supporting Information, everything is there, from zero percent yield to 99 percent. That’s really helpful, also because it’s really good for checking reproducibility.’ A final benefit is modularity. ‘We have a flow reactor coupled to it, but if you have a different type of reactor or capillary, you can swap it out and RoboChem can then do the optimisation process specifically for your very own chemistry.’
Slattery, A. et al. (2024) Science 383(6681), DOI: 10.1126/science.adj1817














Nog geen opmerkingen