Matthias Bickelhaupt’s group (VU Amsterdam) turns yet another generally accepted ‘fact’ in (physical) organic chemistry on its head: methyl radicals are not stabilised by more substitutions, but rather destabilised. They provide the proof in Angewandte Chemie.
Previously, we wrote about paradigm shifts in the bond length and bond strength of C-H bonds, but Bickelhaupt’s group has not been idle. They are now bringing evidence against a well-known rule of thumb in organic chemistry, namely that the more substituted a methyl radical is (in the form MemH3-mC∙, with m = 0-3), the more stable it will be. This would consequently be the reason for the weakening of the C-H bond of the MemH3-mCH alkane at higher substitution. Eva Blokker and colleagues at the Vrije Universiteit Amsterdam provide evidence that the first claim is incorrect, while the second claim holds.
Bickelhaupt had been walking around with this idea for some time, PhD student Eva Blokker tells us. ‘He always had the idea that methyl substituents on carbon radicals would also lead to steric repulsion of the radical, which would therefore make the radical less stable.’ The textbook explanation that a more substituted radical would be more stable seemed to be wrong in Bickelhaupt’s eyes.
He decided to test this idea with his team. ‘We looked at both sides of the reaction’, Blokker explains. ‘So both the parent molecule and the formed radical, and all the steps in between. We broke them down with DFT calculations (M06-2X/TZ2P, ed.) and energy decomposition analyses and looked at the stabilisation or destabilisation of the various steps in the reactions.’ See also the scheme below. What the observations show is that the alkane and the corresponding radical follow the same trend: the more methyl substituents, the more destabilised both molecules are, with the radical being less destabilised than the original.
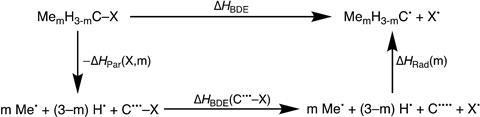
The reason behind the destabilisation is simple, according to Blokker: ‘The bond to a methyl group is weaker than the bond to the original hydrogen atom, and in addition there is steric repulsion between the methyl substituents. The reason that the destabilisation is greater in the alkane is because it is more sterically dense (coordination number of the central carbon is 4), so the increase in methyl substituents directly results in more destabilisation.’
The relevance of this research lies first of all, of course, in the fundamental knowledge about radicals, but this knowledge can also help in a practical sense. Blokker: ‘In my PhD project, we are working together with chemical company Nouryon. We think along about polymer chemistry; specifically, we design new polymerisation initiators for them. If you want to have a radical that is as reactive as possible, it is useful to know something about the instability.’
The next textbook page that Bickelhaupt’s group is slowly tearing out is about captodative radicals. Want to stay up to date? Then make sure you receive our newsletter and keep an eye on our website!
Blokker, E. et al. (2022) Angew. Chem. Int. Ed., e202207477, doi.org/10.1002/anie.202207477












Nog geen opmerkingen