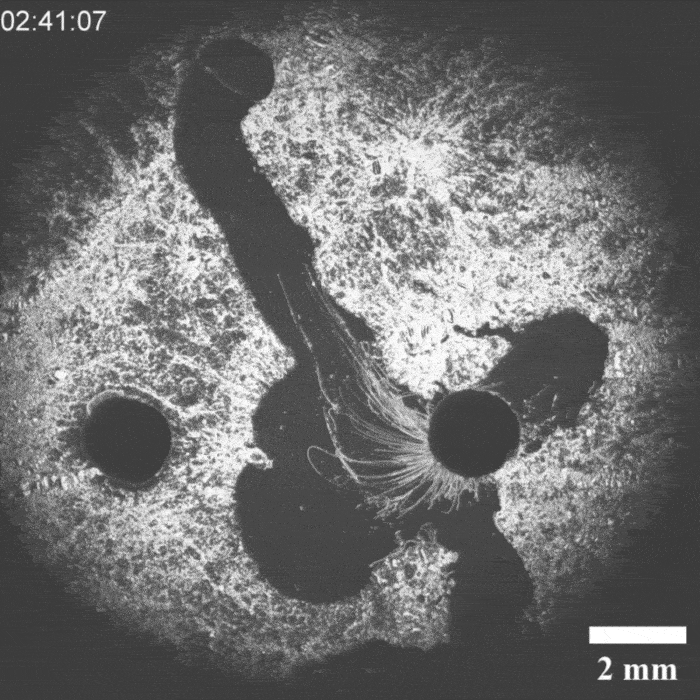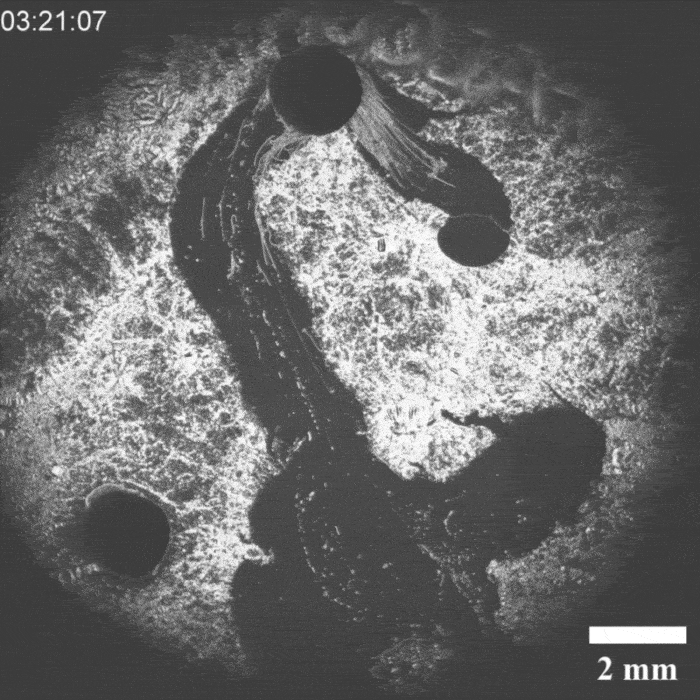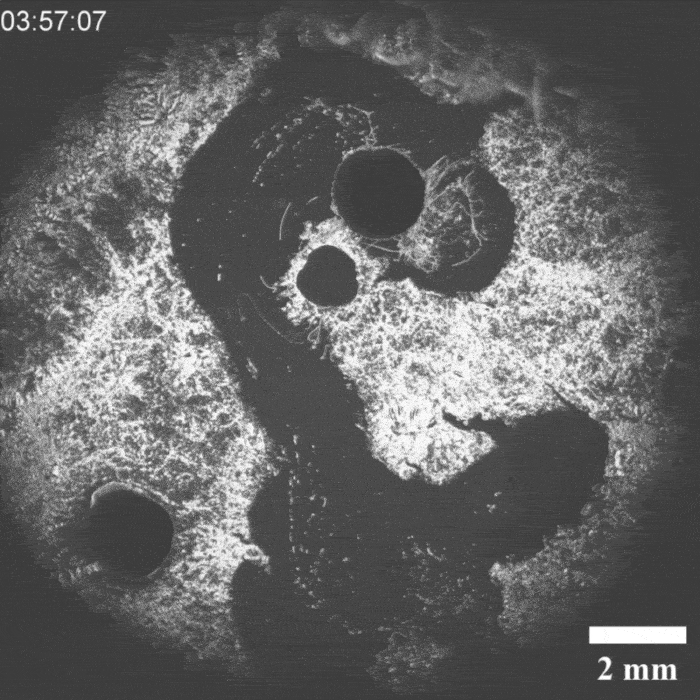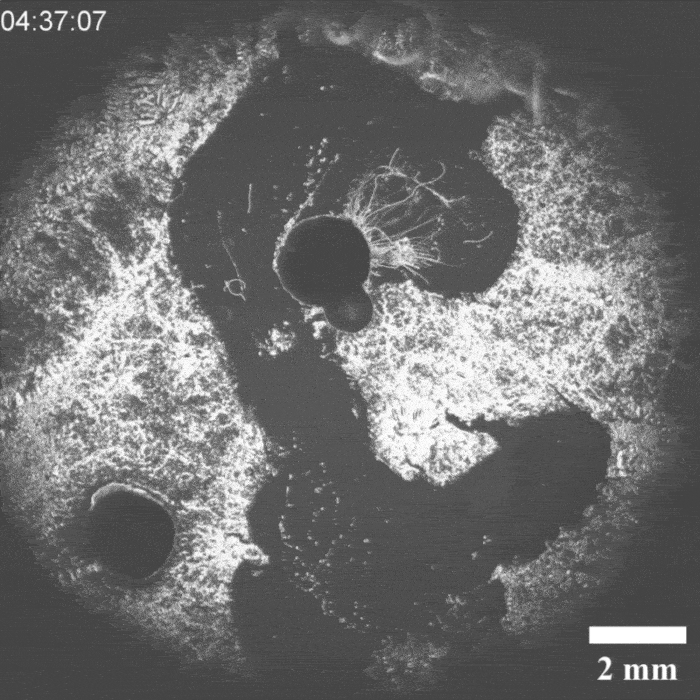
It was a hit on LinkedIn: a moving droplet being chased by another droplet, which then ‘catches’ it. This experiment by Peter Korevaar’s group provides insights into physico-chemical processes that can be found in everyday life, they write in Angewandte Chemie.
In many cases, chemistry is an ‘invisible’ science: reactions between molecules are often impossible to see. But sometimes it is possible – at least indirectly – at the macroscopic level. ‘I posted a video of our research on LinkedIn and it has been viewed many times’, says Peter Korevaar, assistant professor of physical organic chemistry at the Radboud University. ‘It’s great fun to be able to visualise chemistry in five seconds. As a chemist, you’re used to relying on NMR or other indirect methods, but in our case you can actually see what’s happening.’
Korevaar’s group works on the self-assembly of chemical systems at the mesoscopic or macroscopic level. ‘We are fascinated by life, so we try to create life-like materials using relatively simple molecules and known chemical reactions. How do physico-chemical principles such as surface tension or osmosis lead to self-assembly in a way that can be observed with the naked eye?’
Prey-predator pair
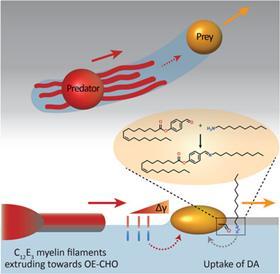
Priyanshu Singh, the PhD student who brought the project to life, discovered the moving system by chance, says Korevaar. ‘We were applying a layer of decylamine to a water-air surface, but it crystallised unintentionally. Then we saw oil droplets moving through this layer and thought: what’s going on here and, more importantly, can we manipulate this?’ According to Korevaar, you can draw a comparison with a white blood cell chasing a bacterium. ’It is very funny to “accidentally” recreate something like this synthetically with very simple molecules. It’s quite typical of a lot of research in my group.
It took a while before they knew exactly what was going on, but it turned out that an oil droplet with an aldehyde group that they introduced reacted with the decylamine and ‘ate’ a piece of it. If you then add an ether-based droplet, it follows the trail of the previous droplet through the decylamine layer, so you could see the two droplets as a kind of prey-predator pair.
Buttons
‘The biggest obstacle we then faced was coordinating all these interactions’, explains Korevaar. ‘You want to prevent the prey droplet from moving too slowly or too fast. The predator chasing it must also have the right speed.’ In the end, they managed to control all these parameters.
With this fairly fundamental research, Korevaar wants to show how lifeless molecules can be used to achieve things seen in life. ‘You can come up with ideas on paper, but when you do it experimentally, you see what buttons you have to push and what reactions you have to have. That’s where you see Priyanshu’s strength and creativity. He has thought very carefully about which reactions you can use to move the droplet on such a crystalline surface.’
Chemical Computer
In follow-up research, Korevaar wants to take steps towards cascades. Korevaar: ’How wonderful would it be if droplets met and reacted with each other to create a new effect?
But you could think bigger: take a field with lots of these droplets. Each droplet then becomes an input to a system. ‘The final movement of the combined droplets then forms a superposition of all the chemical inputs you have put in’, explains Korevaar. ‘You can think of it as a chemical computer that not only performs calculations, but also immediately produces something as a result of those calculations.’ Where a normal computer works with bits and bytes, here self-organisation becomes the programming language. ‘This is really one of the practical possibilities, not only to create a chemical programme in silico, but also “in materia”.’
‘You can imagine that in such a system you have to link typical chemical reactions to real diagnostic reactions that you want to perform, such as links to proteins, DNA and so on’, Korevaar continues. ‘These are future stages that we have to think about further. But once you have the physical chemistry under control, you can really take new steps.’
Singh, P. and Korevaar, P.A. (2025) Angew. Chem. Int. Ed. e202502352, DOI: doi.org/10.1002/anie.202502352












Nog geen opmerkingen