Thanks to a nickel complex, Utrecht researchers have discovered a new H2 activation mechanism that they can follow experimentally, they write in Nature Chemistry.
Hydrogenation – a (catalytic) reaction in which you couple molecular hydrogen to unsaturated organic compounds – has applications in the margarine and petrochemical industries, and the reaction is also regularly found in the laboratory. However, H2 has to be activated first, and this is usually done with the later transition metals. The disadvantage is that they are scarce and expensive. María Sansores-Paredes, Martin Lutz and Marc-Etienne Moret from Utrecht University have now developed a nickel complex that provides a new hydrogen activation mechanism.
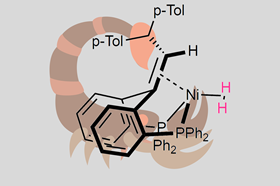
The nickel atom in this complex is coordinated to a so-called ‘pincer’ ligand. Two phosphine groups hold the metal in place. In addition, the ligand contains a double bond in a kind of scorpion-tail configuration, which is also coordinated to nickel. ‘The idea of using a pincer ligand with an olefin was a deliberate choice, but our first designs were not successful’, says Marc-Etienne Moret, associate professor of organic chemistry at Catalysis in Utrecht.
He explains that the complex from the paper was a stroke of luck, based on work from a few years ago. ‘When I saw one of the degradation products from our older work, I thought it might be a good candidate for hydrogen activation. That turned out to be right.’
Surprise
‘The remarkable thing is that the complex not only activates hydrogen but also acts as a catalyst’, says Moret. ‘What is more, during the catalytic reaction you can follow what happens to the catalyst itself, which is often difficult to do.’
The activation mechanism also came as a surprise. Moret: ‘The mechanism had previously been proposed on the basis of DFT calculations for C-H activation, but not for H2 activation.’ In the published system, you see the two intermediates interacting experimentally. ‘This has not been shown before.’
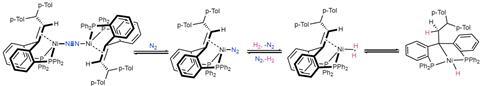
It works like this: first, a hydrogen molecule coordinates to the nickel atom. Nickel pulls the hydrogen atoms apart, then one stays on the nickel and the other moves to the ligand alkene. Then, when an alkyne substrate coordinates to the nickel atom, nickel reduces the alkyne to an alkene with the hydrogen molecule that has been pulled apart.
Tricky gas
One of the experimental challenges was to characterise the nickel-hydrogen complex. ‘We saw a small bump in the NMR spectrum and suspected that this was the complex, but of course this is not sufficient proof’, says Moret. To confirm this, the team carried out experiments with HD (deuterated hydrogen). ‘This is not easy: the catalyst is sensitive to air and water, and HD is quite expensive, so you have to be careful. It was a clever piece of work by María.’ Combined with DFT calculations, the team was able to confirm the catalytic mechanism.
Moret hopes that this work will be a stepping stone to practical applications. ‘The system from the paper will probably not be used by anyone because it is too complicated to make. But a PhD student has just started a new project with us based on this work, who wants to make this kind of complex in a simpler way and will be looking at reaction mechanisms.’
Moret is not entirely sure, but ‘you should always have hope’, he believes. ‘It is difficult to predict the future, but because this is a new mechanism, we expect that this type of complex will catalyse reactions that were not possible before. If this is the case, we can really start applying them.’
Sansores-Paredes, M.L.G., Lutz, M. & Moret, ME. (2024) Nat. Chem. 16, DOI: 10.1038/s41557-023-01380-1












Nog geen opmerkingen