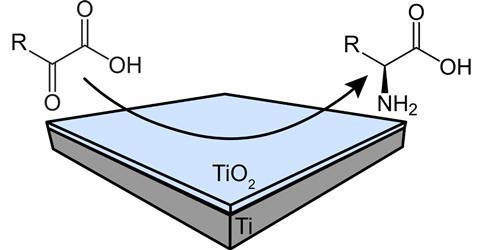
With a combination of flow and electrochemistry, you can synthesise the amino acid alanine in a greener way than by fermentation, a team from Twente writes in ChemPlusChem.
Amino acids are not just the main components of the proteins and enzymes in your body, they can also be used in food supplements, pharmaceuticals and many other applications. The current method of producing amino acids is via organic synthesis or microbial fermentation, which are high-energy and at times complex processes that require harmful chemicals. Christos Englezos, Akash Raman, Arturo Susarrey, and colleagues from the University of Twente studied an alternative that uses an electrochemical flow reactor to synthesise alanine in a greener way.
‘Amino acids like alanine have many applications, especially in food additives’, says Christos Englezos, a former master’s student in Mesoscale Chemical Systems where the research was carried out. ‘We wanted to find an alternative to the fermentation method while incorporating renewable feedstock in the form of raw biomass.’
‘The electrification of these synthesis methods is key for the green transition’, explains Akash Raman, postdoc in the same group. The team used a zero-gap flow electrolyser with a titanium/titanium oxide catalyst combination. ‘We designed a zero-gap electrode in such a way that there was no gap between the electrodes and the membrane. This reduces the resistance between the two electrodes which is important for organic electrochemical reactions.’ Raman further notes that if you use green energy to run the reactor, it will be a sustainable alternative to conventional organic synthesis or fermentation.’
An important aspect to consider is the porosity of the electrodes. ‘The reaction mixture flows through the porous layers of the catalyst’, says Englezos. ‘This way it’s going through a web of fibres. The more porous the material, the higher the active catalyst area where the reaction takes place.’ Specifically, TiO2/Ti felt electrodes with a thick and porous oxide layer showed the highest conversion efficiency – highlighting the importance of the oxide layer thickness and the role of the oxide in the reaction mechanism. The team achieved an optimal alanine Faradaic efficiency of 75% at 2.0 V applied cell potential and 10 mL/h reactant flow rate.
One of the challenges for the team was the electrode itself. Raman: ‘When we first attempted the synthesis, the electrodes didn’t work as expected. As organic electrochemistry is a relatively new direction for our lab, we had a lot of learning to do.’ In the end, the role of the electrode itself became a focus of the study and after some tinkering and experimenting, the team succeeded.
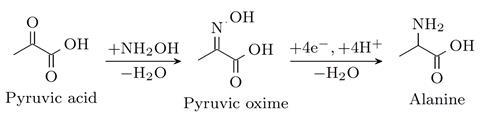
Another challenge concerned determining how much alanine was formed. ‘We got a mixture of product, an intermediate and other molecules’, Englezos explains. ‘Eventually, we found that NMR spectroscopy was the way to go, and we could comprehensively prove that our method works.’
Before this alanine production method can be applied at an industrial scale, further optimization is needed. ‘A key drawback of our method is that it’s not enantioselective, meaning we create both D-alanine and L-alanine’, says Raman. ‘Only L-alanine is compatible with life.’ Englezos adds: ‘We also need a way to purify the product, which is important for scaling up.’ In addition, the reaction the team studied is only one step in the conversion of biomass into amino acids. ‘The other steps need to be studied and made sustainable too’, says Raman.
The team has also looked into how the reaction would fare if you would use more than one precursor. ‘But we need to optimize our protocols’, says Englezos. ‘It seems like something was inhibiting the conversion when we used a mixture of two precursors.’ It is an important next step according to Raman. ‘When you start the process from raw biomass and break it down, you automatically get a mixture of different precursors and this can lead to the production of a mixture of amino acids, which would kill multiple birds with one stone, so to speak.’
Englezos, C. et al. (2024) ChemPlusChem e202300763, DOI: 10.1002/cplu.202300763












2 Opmerkingen van lezers