An international team has discovered that an eight-membered ring has a previously overlooked potential for chirality. Its surprising robustness opens up interesting applications, they report in ChemPhotoChem.
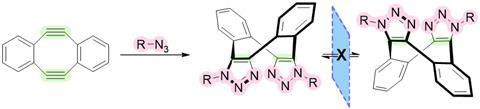
‘The study of chiral eight-membered rings dates back to the 1960s’, says Donal O’Shea, Professor of Chemistry at the Royal College of Surgeons in Ireland. ‘At that time there were some successful syntheses, but most were not sufficiently stable for isolation of individual enantiomers.’ In the 1990s, researchers using tetra-aryls as substituents found that they greatly increased their stability, allowing enantiomer separation and isolation. ‘In our work, we were initially interested in making dibenzo-cycloocta-bis-triazoles inside living cells through two sequential azide cycloadditions from commercially available dibenzo-cyclooctadiene-3,7-diyne.
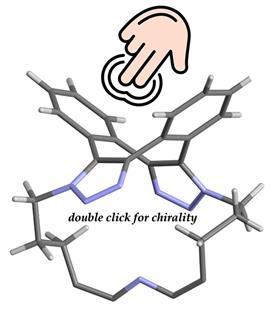
But while working with these molecules, O’Shea and colleagues from Newcastle University and the University of Antwerp discovered the potential for chirality in this eight-membered ring, ‘a form that has been overlooked’, as the article states. ‘You can see that it’s there, but the challenge was to experimentally prove it’, says O’Shea. To help in this quest, they made a fluorescent macrocyclic derivative using a BF2-azadipyrromethene dye. This not only showed the chirality, but also that this chirality was of great value.
Long collab
O’Shea explains: ‘Thanks to its easy synthesis and robust chirality, you can imagine many applications of this scaffold, especially where you want to use only one enantiomer, such as in medicine or catalysis.’ O’Shea’s group is particularly interested in looking at the chemical processes in cells. Fluorophores with electronic circular dichroism (ECD) and circularly polarized luminescence are increasingly sought after due to their potential applications for optical data storage and encryption, display devices and biological probes, he elaborates. ‘Our current focus is to look at and track this double alkyne-azide transformation in specific subcellular regions, ultimately including its enantioselective reactions.’
The present work is the result of a long collaboration, says Wouter Herrebout, professor of chemistry at Antwerp. Herrebout and Dmitry Levshov (also from Antwerp) were responsible for the experimental ECD measurements and DFT calculations of the molecule. ‘We previously worked on chiral BODIPYs with Michael Hall from Newcastle [also one of the authors, ed]. This story is basically a continuation of all the work we did on chromophores before.’
Straightforward
Although the macrocycle looks quite complex, the dibenzo-cycloocta-bis-triazoles are quite easy to make. ‘It is a fairly straightforward reaction under mild bioorthogonal conditions at room temperature’, says O’Shea. But separating the enantiomers – and then getting enough material – was very challenging, especially for the important fluorophore derivative. This had to do with the type of chromatography the team performed. ‘They used preparative chiral chromatography, which takes a lot of time’, says Herrebout. ‘In addition, the amount of sample we got after the separation was less than we expected. It was barely enough for analysis.’
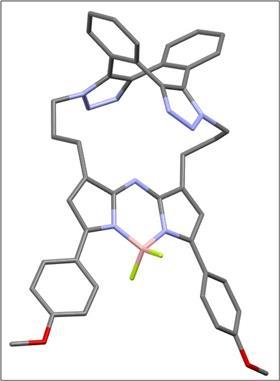
The two Newcastle teams (Mike Hall and Roly Armstrong) were able to crack the enantiomer separations and make it work, O’Shea acknowledges, ‘with Michael Hall being the central point between all of us. When I came to him with our separation challenge, he almost immediately had a plan to resolve and study the photophysics of the enantiomers. All in all, it was a really big team effort.’
Nightmare
Another challenge was the quantum calculations. The Antwerp team was responsible for determining the absolute configuration. ‘It took a bit longer than usual’, Herrebout clarifies. ‘The previous chiral BODIPYs we had worked on were relatively small molecules, but this one was quite large, which required more calculation time.’ Although there wasn’t a one-to-one match between the simulation and the experiment, the Flemish researchers were able to assign the absolute configuration. ‘We waited a long time to see if we could get a better spectral prediction, but it turned out to be an absolute nightmare to get this perfect, so we went ahead and published the article.’
In addition to the practical side of this molecule, Herrebout says it also has some theoretical value. ‘It serves as a nice teaching example because it has C2 point group chirality despite its rotational axis. Usually that’s seen as a limiting factor for chirality, but this shows that’s not the case.’ Normally you would see applications flowing from theory, but here it seems to be the opposite.
Pim, S. et al. (2025) ChemPhotoChem e202500042, DOI: 10.1002/cptc.202500042












Nog geen opmerkingen