Samuel Stupp designs and develops supramolecular polymers for various applications, ranging from clean energy technology to regenerative medicine. But his polymers are most famous for enabling mice to use their legs again after a spinal cord injury.
From the downtown Chicago office of Samuel Stupp, professor of Materials Science and Engineering, Chemistry, Medicine, and Biomedical Engineering at Northwestern University, you look out onto the Northwestern Memorial Hospital. An inspiring view, if you ask Stupp, who likes to combine scientific fields and cross the boundaries of chemistry to achieve what he set out to do: making a real impact on people’s life.

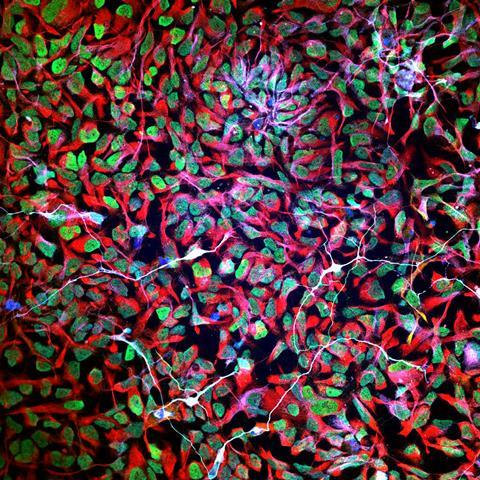
Everything in Stupp’s research is based on self-assembling polymers. In his lab on Northwestern’s primary campus in Evanston, he looks for ways to generate clean energy, for instance with polymers that produce hydrogen and other fuel molecules using visible light or that convert light to mechanical energy in robotic materials. Here at the medical center, his focus is on regenerative medicine. ‘In the early nineties we made a molecule that serendipitously formed a two-dimensional supramolecular polymer, and since then I have been interested in understanding self-assembly and how molecules in these structures interact’, Stupp explains. ‘Two of their features are hierarchical structure and complex dynamics, something we also see a lot in nature, including our own bodies. So, it seemed like a logical step to combine the fields and look at regenerative medicine.’
‘We can now tune the molecules very precisely and make them suitable for regeneration of lots of different tissue types’
Peptide amphiphiles
About twenty years ago, his research led to the development of peptide amphiphiles; the molecules that Stupp still works on. These molecules are composed of amino acids and other biomolecular structures such as lipids, sugars and nucleic acids. Stupp: ‘They were designed to be self-assembling and biodegradable, and we also wanted to incorporate bioactive signals in the structures so they could interact with receptors in the body and influence processes there, like the growth of a certain tissue.’ When put in water, the peptide amphiphiles can form filaments – strings with hydrophobic lipid tails pointing inward and bioactive molecules pointing outward. ‘But it is also possible to get different shapes, like twisted ribbons, by tuning the molecule.’
The first time Stupp and his team created peptide amphiphiles, back in 2001, they did not really understand what was happening in their self-assembled structure. But since then, they gained a lot of knowledge, he says: ‘We can now tune the molecules very precisely and make them suitable for regeneration of lots of different tissue types.’ Bone, cartilage, muscle, enamel; with the right biological signal molecule in place, they can make all these tissues grow. Stupp: ‘We can now even use them to promote the growth of blood vessels.’
Injecting the fibers
The first step to all this regeneration is to create the structures. When the peptide amphiphiles are placed in water, fibers form in less than a second. If you want to make the fibers soluble in water, you can introduce charges in the molecules. ‘This property allows us to inject the molecules into the body’, Stupp explains. ‘Our body contains a lot of salts, so when we inject the solution into the exact place we need it, the salts shield the repulsion among charges and a hydrogel forms almost immediately.’
It is even possible to suppress the formation of the hydrogel by diluting the solutions of fibers. ‘A few years ago, we found that very dilute solutions of the peptide amphiphiles can be put into the bloodstream. If we then incorporate a specific molecular segment we can target a specific location of the body, for example to deliver a drug or to form a “hydrogel band-aid” to stop internal bleeding.’
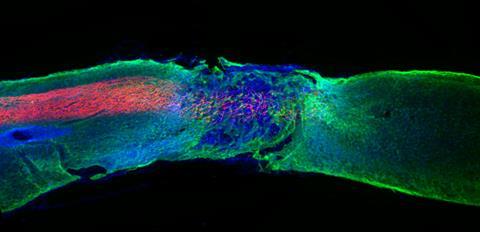
Movement regained
One of the most exciting finds was the discovery that the amphiphiles could also be used for healing spinal cord injuries. ‘We injected specially designed peptide amphiphile fibers with regenerative signals into severely injured spinal cords of mice’, Stupp explains. ‘After twelve weeks, we saw that the mice regained movement in their hind limbs. This was very exciting.’ Last year the researchers gained a crucial insight, being that the dynamics of the molecules in their systems are key to the observed regeneration. ‘The receptors of cells are in constant motion’, Stupp says. ‘To increase the chance of our biosignals meeting these receptors, our system had to move as well. So, we designed our peptide amphiphile molecules to be dynamic within the fibers.’
‘We saw much more growth of axons in neurons, regeneration of blood vessels and other improvements’
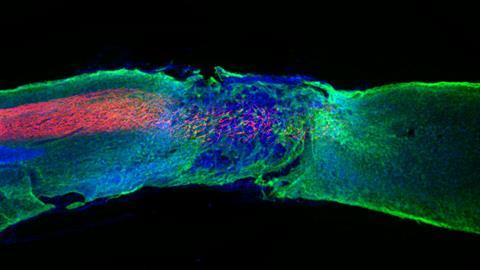
Beneficial dancing
The key turned out to be a different set of amino acids, which caused the interactions between molecules to become weaker, and thus the system to be more dynamic. Stupp: ‘Individual peptide amphiphiles can now move above the fiber surfaces, move within the fibers, or sometimes even jump out of the structure temporarily and then return to the filament.’ These dynamic systems, which seemed to be dancing, turned out to greatly benefit the regenerative properties. ‘With the dynamics, we saw much more growth of axons in neurons, regeneration of blood vessels and other improvements’, Stupp explains enthusiastically. ‘This led to significantly better movement in the mice after just four weeks. I don’t say it often, but this felt like a real breakthrough.’
With this knowledge and the platform in place, the next step is clinical development. It is hard to predict if the positive results will also be achieved in humans, but Stupp is optimistic. ‘I have hope it will also work in humans. But there are of course still hurdles to take. For instance, in mice we injected the molecules within 24 hours of injury, so we don’t know if it can also help chronic injuries.’ From a scientific point of view, many questions remain. ‘I want to understand supramolecular structures and the underlying mechanisms for their dynamics as deeply as I can, so we need to develop more molecules and test more systems.’ Stupp is often invited to speak at spinal cord injury meetings where patients participate. This is very motivating, he says. ‘These people have hard lives. I want to help them and others as much as I can. So, I will keep working until I succeed.’
This article was created with support of the ’Trip Fonds’, which is managed by the VWN, Association for Science Journalism and Communication Netherlands.












Nog geen opmerkingen