A team of Eindhoven- en Nijmegen-based scientists explains in Nature Biomedical Engineering how they succeeded in targeting interleukin-4 to immune cells in the spleen and bone marrow. That offers the prospect of a therapy against sepsis and other life-threatening conditions associated with hyperinflammation and immunoparalysis.
Interleukins regulate the immune response through either pro- or anti-inflammatory activity. Thus, anti-inflammatory interleukins are expacted to have a dampening effect on trained immunity, also known as the ‘memory’ of our innate immune system. Thanks to this relatively recently discovered phenomenon, first described by professor Mihai Netea of the Radboud University Medical Center in Nijmegen (The Netherlands), the innate immune system remains on standby after a first trigger, resulting in a much faster and more intense immune response when triggered a second time.
Paradoxical
Following this reasoning, anti-inflammatory effects and trained immunity are opposite effects. It therefore came as a big surprise when Rutger Röring, PhD student in Netea’s group, discovered that interleukin-4 (IL-4) both inhibited inflammation ánd activated trained immunity in primary human monocytes (white blood cells).
This paradoxical character of IL-4 was not just nice to know, it also immediately sparked ideas for a potential clinical application. ‘In people suffering from severe infections, immunoparalysis is often seen as well,’ explains professor of precision medicine Willem Mulder (Radboudumc/Eindhoven University of Technology). ‘Persistent inflammation “paralyses” the immune system, as it were, so that no immune response is initiated. This is a major clinical problem, because you can’t really do anything to counter this and as a result, patients often die from another, opportunistic infection, such as pneumonia.’
Docking
IL-4 could therefore, in theory, simultaneously attenuate hyperinflammation and ‘kick’ the immune system back into action. But will only happen when IL-4 reaches the necessary immune cells, i.e. the monocytes, which implies that it has to be delivered to the spleen and bone marrow. Mulder: ‘That delivery was our problem. In vitro it works, but in a living organism you cannot just direct IL-4 to the myeloid cells [the precursors of monocytes, ed.]. Using a standard lipid nanoparticle as a delivery vehicle doesn’t make sense, because IL-4 is then absorbed by the cell. With our fusion protein technology where we couple IL-4 to the body’s own apolipoprotein A1, we force an interaction between IL-4 and the target cell’s surface. ApoA1 docks on the surface of myeloid cells and that enables IL-4 to bind to the relevant receptor.’
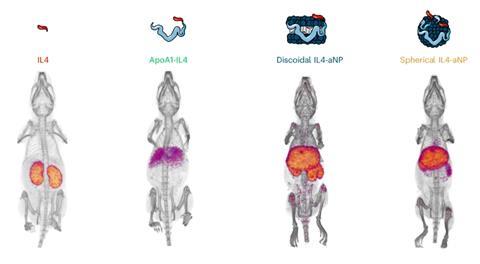
Studies in mice clearly showed the effect of ApoA1 and nanoparticle packaging on biodistribution. ‘Naked’ IL-4 and ApoA1-IL-4 mainly ended up in the kidneys and liver, respectively, while the packaged versions in discoidal or spherical nanoparticles actually accumulated in the spleen and bone marrow. Follow-up studies in non-human primates also showed desired uptake and a good safety profile. Mulder: ‘IL-4 is species-specific and human IL-4 does not work in mice. Therefore, to get a realistic picture of its safety in humans, you cannot solely rely on studies in mice.’ And then there is the question whether packaged IL-4 actually produces a beneficial effect. In a modified mouse model, this seems to be the case. The activity of the immune response increased, while hyperinflammation decreased.
Infarct
Clinical studies in humans are the next step. According to Mulder, there are plenty of conditions for which IL-4 therapy may be relevant. ‘You often see the combination of hyperinflammation and immunoparalysis in sepsis. There doesn’t seem to be much interest in sepsis, but it leads to around 3,500 deaths each year in the Netherlands. In addition, hyperinflammation and immunoparalysis are also encountered in patients struck by a myocardial or cerebral infarction. Those patients often die of hyperinflammation. The infarct causes an overreaction and the whole system get derailed.’
Looking back, Mulder is very pleased with the way the whole project turned out. ‘I really think this is a textbook example of synergy and team science. Four years ago, our first PhD student David Schrijver started working on this fusion protein technology. Because of the collaboration with immunologists from Radboudumc, all aspects from basic biology and immunology to technical application and translation are covered in this study. Mihai and I also made a conscious decision not to publish all the individual results in smaller form, but rather to go through the whole process, so that we could come up with a really good paper.’












Nog geen opmerkingen