In Nature, Chinese researchers propose organocatalysis as a way to make the old but still vital chlor-alkali process more efficient. Selectivity and activity are promising, but long-term stability is a concern.
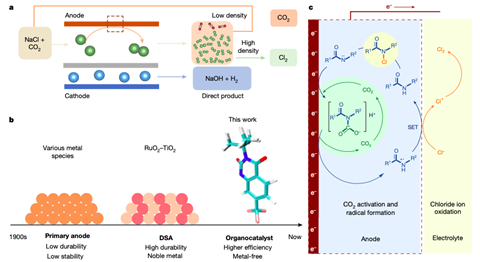
One of the oldest and most fundamental processes in the chemical industry is the chlor-alkali process: electrolysis of a salt solution produces chlorine (Cl2) and sodium hydroxide (NaOH). Both products are in turn the basis for numerous other processes, so the production volumes are immense. Add to this the fact that the chlor-alkali process consumes energy (about 1% of the world’s electricity production) and it is clear that any improvement in efficiency can immediately lead to huge savings on many fronts.
A team led by Dingshen Wang and Yadong Li at Tsinghua University in Beijing focused on the catalyst as a starting point for improving efficiency. Specifically, organic alternatives to the usual ruthenium- or iridium-based catalysts. This has been tried before, but without impressive results. Wang, Li and colleagues now present a new option in Nature: RCON-H, in which the amide group provides the desired functionality. In this process, the supply of CO2 is essential to ensure conversion to NCOOH, and this intermediate provides an exponential increase in catalytic activity.
Selective, active and cheap
The new organocatalyst is on a par with traditional metal-based catalysts, says Thomas Turek of Clausthal University of Technology in an accompanying News&Views article. Both selectivity and activity are extremely high, and in the latter respect RCON-H even outperforms the metal-based competition, says Turek. This alone makes it an attractive option for making the chlor-alkali process more efficient, but a major advantage of organocatalysts is that they can ‘simply’ be produced from cheap, renewable raw materials rather than relying on the mining of expensive, non-renewable (precious) metals.
But the step towards industrial application is still a long way off, says Turek. In particular, the stability of the electrodes with the organocatalytic coating needs to be significantly improved. In this study, it starts to degrade after 450 hours, whereas industry requires a lifetime of at least five years. Turek’s concerns are shared by Bert Vreman, a researcher at chlorine manufacturer Nobian (formerly AkzoNobel). ‘The industry is very interested in research to further improve the efficiency of the chlor-alkali process. Research into long-term stability is important for the application of new catalysts. According to the results in the article supplement, the amount of organocatalyst on the electrode appears to have decreased by several percent after 450 hours. Good long-term stability is very important and has not yet been demonstrated. More research and development is needed before large-scale application.’
Nevertheless, Turek believes that the possibility of organocatalysis is worth investigating further. At the very least, he says, it shows that the assumption that organocatalysts are much less suitable for electrochemical applications than metal-based catalysts is not true. Vreman also recognises the value of this work. ‘It is a surprising paper. It is interesting to see and follow the developments.’


















Nog geen opmerkingen