How hydrogen bubbles form and behave on electrodes seems to depend on the anions in your electrolyte, researchers explain from Leiden and Twente in Nature Chemistry.
The large-scale production of hydrogen by electrolysis is really starting to take off. But to use your electricity as efficiently as possible, it is important to know what is happening at your electrodes. Ideally, you would use high current densities, but that creates bubbles at your electrode and these create resistance. ’This is a very old topic in electrochemistry: how do bubbles behave in this context?’ says Marc Koper, Professor of Electrochemistry at Leiden University.

’In classical electrochemical research, we usually use a lower current density, with a lower overpotential’, explains Koper. ’In this case, you don’t see bubbles forming, although they could be there. But at higher current densities, you get big bubbles, and bubbles create more resistance on your electrode and therefore lower efficiency.’ A few years ago, Henry White of the University of Utah started researching bubbles using micro- and nanoelectrodes. The advantage of such tiny electrodes is that you can see how a single bubble forms. ’I found this research so attractive that I started doing it myself’, Copper says.
Influence of the electrolyte
’The field of bubble dynamics is populated by engineers and physicists who sometimes overlook the chemistry behind the processes’, Koper continues. ’As a result, certain aspects of bubble formation remain underexplored. We have been studying the influence of the electrolyte on catalysis for a long time, but recently we started to look at the influence on bubbles. Henry White was mainly looking at the nucleation of these bubbles, but I felt that the electrolyte would have an influence on bubble confluence and removal.’
‘You can play with the electrolyte and thus adjust the bubble size to your liking’
At the beginning of the research, a postdoctoral fellow from South Korea, Sunghak Park, looked for a project in Koper’s group, which had just discussed an idea with the fluid dynamics group of Detlef Lohse and Dominik Krug at the University of Twente. Koper: ’It was a great opportunity and Sunghak turned out to be the perfect person: he is very systematic and moves easily between the physics and chemistry of bubble formation. His initial studies showed that the electrolyte did indeed have a major influence on the behaviour of the bubbles on the microelectrode.’
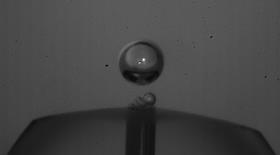
Hofmeister
If you keep all the facets the same but change the acid in the electrolyte, you get a different electrochemical pattern for each anion in the form of oscillations. ’We watched the bubble formation with Detlef and Dominik’s high-speed cameras’, Koper says. ’What we saw on the electrode surface was a kind of blanket of bubbles feeding a larger bubble [see image above, ed.]. In sulphuric acid, you can see a large bubble forming before it leaves the surface, but in perchloric acid, the escaping bubbles are much smaller.’
This difference in bubble size seems to follow the Hofmeister series, a well-known series in biophysics and chemistry, which says that the surface tension and properties of proteins depend on the ions in the electrolyte. ’If you make electrolytes with different differential surface tensions according to the Hofmeister series, the bubble formation follows this series almost exactly’, Koper says.
Text continues after image
Marangoni effect
This led to a paper in Nature Chemistry in which they described it as follows: With sulphuric acid, at one end of the series, the bubbles are large. For perchloric acid, at the other end of the series, the bubbles are small. Koper: ’Because of the electrochemical proton reduction, there is a concentration gradient of protons, and this must be accompanied by a concentration gradient of anions, because of the electroneutrality of the electrolyte. This anion concentration gradient leads to a surface tension gradient. This in turn gives rise to the Marangoni effect [see box below, ed.]. This surface tension gradient induces a current in the electrolyte. This is known as the solutal Marangoni effect, as opposed to the more familiar thermal Marangoni effect.’
In sulphuric acid, the current moves away from the surface and pushes the bubble towards the electrode, causing coalescence and a larger bubble. With perchloric acid, the flow goes in exactly the opposite direction, so the smaller bubbles are pushed away from the electrode, giving them no chance to coalesce. ’You can play with the electrolyte and adjust the bubble size to your liking’, Koper says. ‘A side note is that you shouldn’t make the current density too high. Because then you also get thermal effects, the thermal Marangoni effect, which makes all the electrolytes behave the same.’
How does this translate into practice? Unfortunately it’s not that simple. ‘In “real” electrolysers, the electrodes are in a polymer electrolyte’, Koper explains. ’If you start to look at that, everything will likely be different. But the methods we can use to look at this – as well as electrodes in an alkaline environment – remain intact and can help us discover how things really are. All in all, we are mainly trying to find out how you can influence the formation of bubbles with your electrolyte, and you seem to have a pretty good handle on that.’
Tears of wine
The Marangoni effect is the phenomenon of a liquid moving from a low surface tension to a higher surface tension. In other words, a higher surface tension pulls the liquid ‘harder’ than a lower surface tension. A well known example is the so-called ‘tears of wine’. The mixture of alcohol and water forms a thin layer on a wine glass above the liquid line. Due to the lower surface tension and lower boiling point of ethanol, it evaporates faster than water, creating a concentration difference. This pulls on the rest of the liquid, causing droplets to become too heavy and slide back down, creating a ‘teardrop’ effect.













Nog geen opmerkingen