Making chiral molecules easily is a synthetic chemist’s dream. Francesco Mutti and his team have used modified enzymes to produce amines with two stereocentres in a one-pot synthesis with high precision and efficiency.
It’s no secret that pure chirality in molecules is both very important and difficult to achieve. But the field of biocatalysis has a few tricks up its sleeve in the form of enzymes. Enzymes are natural catalysts and tend to have very specific functions. They work very well together in cells, but it’s a different story in the lab.
‘Whenever you want to carry out a chemical transformation involving several steps at the same time using different enzymes, compatibility is an issue’, says Francesco Mutti, associate professor of biocatalysis at the University of Amsterdam. Recently, however, Mutti and his group have made a significant contribution to the field.
’This paper is really the icing on the cake’

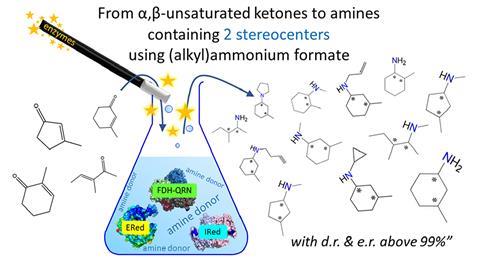
Using modified enzymes, Mutti, senior scientist Tanja Knaus and recently promoted Maria Corrado were able to convert α,β-unsaturated ketones into amines with two chiral centres, with high chemical purity and specificity (both >99%). ‘Until now, no one has really managed to develop a method that is widely applicable’, Mutti explains. Two chiral centres mean there are four possible ways to synthesise a compound, so you need to be able to steer towards a specific stereoisomer. And that’s exactly what his team did, in a way that is easily accessible to other researchers.
No by-products
Mutti: ‘The chirality obtained is determined by the geometry or electrostatic properties of the active sites of enzymes, combined with the orientation of the substrate.’ Because of these facets, you get just one product with almost no by-products. ‘This is because for a reaction to take place, the substrate must be at a certain distance from the enzyme and the cofactor. Since the active site of an enzyme is itself chiral, you need a variety of enzymes – a toolbox – for the different products. In a sense, it is almost like Lego, where you combine the pieces so that they fit together, and some combinations are impossible.’
‘You can just use the enzyme like any other catalyst, but with more favourable reaction conditions’
The team used a combination of ene reductases (EReds, which reduce a carbon-carbon double bond to a single bond) and imine reductases/reductive aminases (IReds/RedAms, which reduce N=C to N–C). They used NADP-dependent formate dehydrogenase (FDH) and an (alkyl)ammonium formate buffer (HCOONH4/HCOONH3R) as an auxiliary enzyme to recycle the cofactor that keeps the reactions going. The ammonium or alkylammonium formate buffer acted both as an amine donor and as a source of reducing equivalents. With this combination, they achieved a diastereomeric and enantiomeric ratio of over 99%.
Mild conditions
But it wasn’t easy. ‘The complexity of this type of work lies in creating the right biocatalyst’, says Mutti. ‘If you want to use this method, you need an enzyme that is highly active, stereospecific, acts on the right molecule, is stable and so on.’ Interdisciplinarity is key to making these enzymes. Tanja Knaus, a brilliant researcher in my group, has had a very good synergy with one of my PhD students, Maria Corrado (who is currently working at Aspen API, ed.). Tanja’s background is in biocatalysis and enzymology, while Maria is an organic chemist, so they complemented each other perfectly for this work. Together they have co-authored six papers, and this last one is really the icing on the cake.’
‘There seems to be a discrepancy between how different European countries use biocatalysis’
So the initial phase of this research is quite complex, but once you have the right enzymes in your toolbox, the actual reactions are very simple, according to Mutti. ‘You can just use the enzyme like any other catalyst, but with more favourable reaction conditions: you work in water, in air, without pressure and maybe with a little heat. In this respect, it’s easier than pure organic compound chemistry.’
Conservative
Now that the methods have been created, Mutti hopes they will be taken up by other researchers and companies. ‘In a large company where automation is possible, you could produce enzymes of interest in a few months’, he says. ‘And it will only get faster, especially with the advent of artificial intelligence and computational methods. You don’t have to do a lot of trial and error like we did.’ The ability to use these very mild conditions is much more sustainable for creating complex molecules in the pharmaceutical industry. ‘In addition, the increased speed and ease with which drugs can be produced will help a lot of people’, says Mutti.
The associate professor is keen to stress that the potential of biocatalysis is largely untapped. ‘There seems to be a discrepancy between how different European countries use this field. In the Netherlands, the chemical community is still a bit more conservative about catalysis than in Austria, Germany or the UK, for example.’ Some researchers still have prejudices against biocatalysis that were relevant twenty years ago, but are no longer. ‘This is a real shame, because if more people recognised the potential of biocatalysis, industry would become more aware of the field, and I think that would be very beneficial for society.’
Knaus, T., Corrado, M.L. en Mutti, F.G. (2022) ACS Catal. 12(23), DOI: 10.1021/acscatal.2c03052












Nog geen opmerkingen