Specially designed sulphonium salt labels can be used as electron acceptors for radical formation in photoactive electron donor-acceptor complexes, where you do not need metal catalysts, write British researchers in Nature Chemistry.
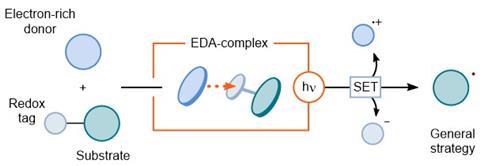
Radicals in organic chemistry are reactive compounds that can engage in unusual reactions. But to form a radical compound, you need to separate the electrons of a bond, and that’s the tricky part. An up-and-coming option is the photoactivation of electron donor-acceptor (EDA) complexes. As the name suggests, this works with an electron donor and an electron acceptor forming a complex. If you then shine (visible) light on the complex, a single-electron transfer (SET) takes place, which results in two radicals.
It works with aromatic compounds (also called arenes), but only if you attach the right functional groups. As such, you have little creative freedom and are limited by the electronic properties of the aromatic precursors. British researchers at the University of Manchester led by David Procter thought that was too inefficient. So they designed a widely applicable method to functionalise specific C–H bonds on arenes with sulphonium salts, visible (blue) light and without metals.
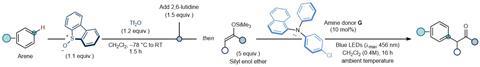
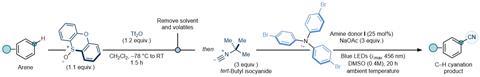
In this new method, you start with non-functionalised arenes. To this you add activated sulphoxide with an amine catalyst. This subsequently forms an EDA complex that will perform a SET under irradiation of visible blue light. The resulting radical is able to react with all kinds of other molecules, such as cyanide-precursors and activated enol compounds, of which the researchers show a host. Besides, you can also vary the arene itself with halogens, electron donating or withdrawing groups. The yields of the reactions were around 50-60%.
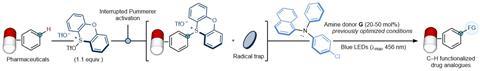
Procter and his group also demonstrated the practical application of this method by using it to either easily remake or modify existing pharmaceuticals or agrochemicals. The authors conclude: ‘We envisage that our EDA-complex photoactivation strategy will serve as a launchpad for the invention of novel organocatalytic aromatic C-H functionalisation processes.’
Dewanji, A. et al. (2022) Nat. Chem., doi.org/10.1038/s41557-022-01092-y













Nog geen opmerkingen