Researchers in Groningen have developed a two-step synthesis that makes it possible to modify cellulose so that it is not only soluble in water, but also easy to functionalise, according to RSC Sustainability.
Cellulose could very well be a substitute for fossil fuels, but then you have to find a way to dissolve it. Most organic solvents are not suitable for this purpose, and water does not work either, despite the large number of hydroxide groups found on cellulose. But with two simple synthetic steps, you can make a form of cellulose that dissolves in water, according to Peter McNeice, Ben Feringa and colleagues at ARC CBBC and Nouryon.
Making cellulose soluble was already possible with the viscose and lyocell processes, but either toxic solvents are needed or the price of the compounds skyrockets. So the team looked for an alternative that would allow them to use commercial reagents to modify cellulose under mild conditions.
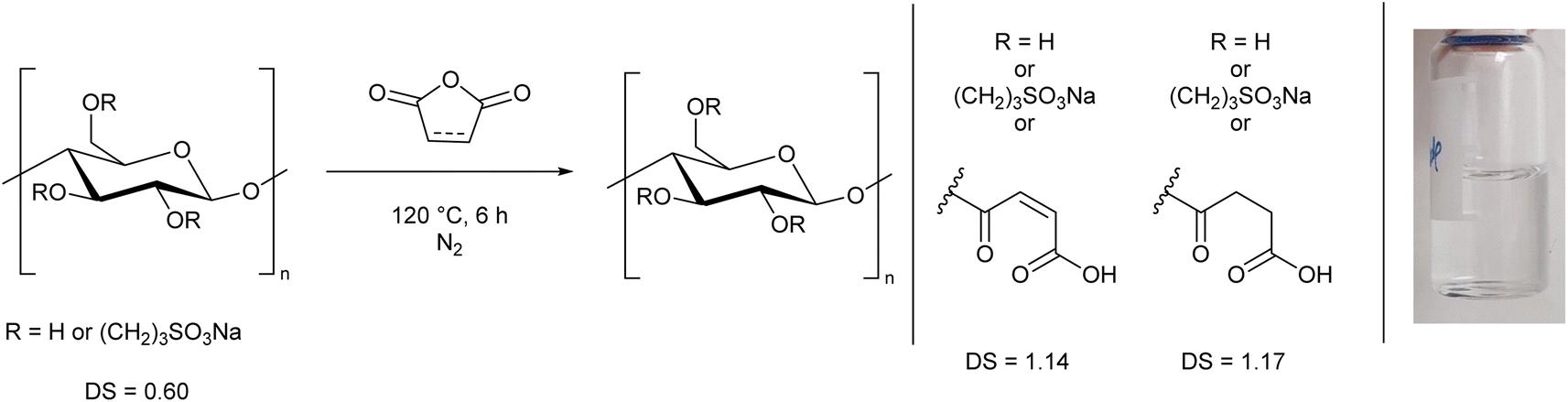
This involves reacting cellulose first with 1,3-propanesultone and then with maleic anhydride or succinic anhydride. After these reactions, in which you have replaced some of the hydroxyl groups, your cellulose derivative is water soluble. It turned out to be essential to incorporate a sulphur group, without which solubility is lacking. They also had to replace at least one hydroxyl group per glucose subunit.
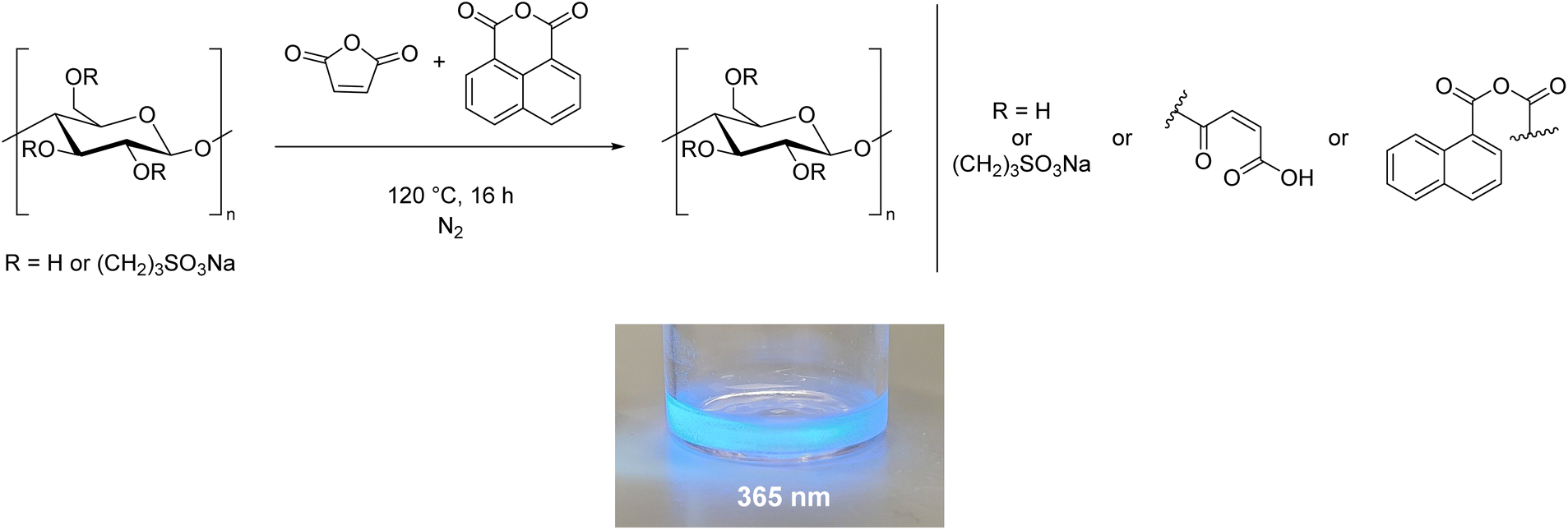
But the researchers did not stop at solubilisation. They showed that it is also easy to introduce other functional groups using this method. As a concrete example, they allowed the sulphonated cellulose to react not only with maleic anhydride, but also with a fluorophore (1,8-naphthalenic anhydride). The blue fluorescent test tube in the paper (see below) shows that the method works.
McNeice, P. et al. (2024) RSC Sustain., DOI: 10.1039/D3SU00317E












Nog geen opmerkingen