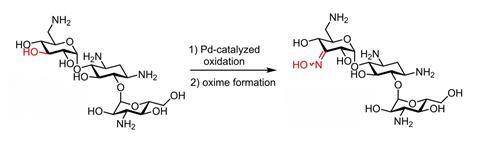
By first protonating the amine group on a sugar, you can selectively oxidise one of the hydroxy groups with palladium without further protecting groups, Dutch and Swiss researchers show in Chemistry A European Journal.
In organic synthesis, you can’t get very far without protecting groups: you need to protect one reactive or sensitive part of your molecule so that another reactive group can react. All this protecting takes time and effort and can also reduce yields. Sugar chemistry also suffers from this, but the groups of Adri Minnaard and Martin Witte at the University of Groningen and colleagues in Zurich have now shown how you can selectively oxidise aminoglycans at C3 without protecting groups.
It was already known that the C3 position could be selectively oxidised with a palladium catalyst. The researchers refer to earlier work in which they did exactly that, but with a protecting group on the primary amine at the C6 position. This is because these side groups behave like a Lewis base when the catalyst is a Lewis acid. So the side group inhibits the catalyst, preventing it from doing its job.
But if you add a Brønsted acid before you add the catalyst, you protonate the amine group and prevent it from reacting with the catalyst. This is what the researchers show in this paper, first with monosaccharides for demonstration, but then also with trisaccharides. Methanol with trifluoromethanesulfonic acid (TfOH) worked best.
The researchers immediately put this method into practice by producing variants of kanamycin A. Kanamycin A, an antibiotic, is a trisaccharide with such a primary amine at the C6 position. Resistance to these types of antibiotics is a recurring problem, so if you can easily make variants of such compounds, you have a better chance of finding a variant that bypasses the accumulated resistance to a compound.
The variants were made by the same synthetic route: you protonate the trisaccharide - with all sorts of unprotected hydroxy and amine groups - with acid and oxidise it with the catalyst. They made all sorts of variants in this way and tested them. While most did not necessarily work better than the original kanamycin A in normal bacteria, they did in mutant bacteria that mimicked resistance.
The beauty of this work is the huge reduction in reaction steps they achieved. The traditional oxidation pathway involves 11 steps - ten of which are protection and deprotection steps and only one step for oxidation. In this new process, there is only the one oxidation step. They also improved an earlier protocol for one of the variants: where they previously had a total yield of 10% over four steps, they now achieved 58% over two steps. All in all, this is an interesting tool for chemical biology and aminoglycoside-based antibiotics.
Marinus, N. et al. (2024) Chem. Eur. J. e202400017, DOI: 10.1002/chem.202400017

















Nog geen opmerkingen