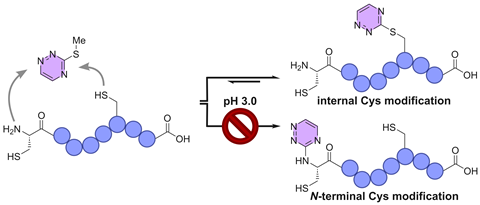
Using triazine-thiol exchange, a group from Nijmegen was able to program cysteine modifications in peptides by varying the pH, they report in ChemistryEurope.
Modifying peptides or proteins for therapeutic applications presents the challenge of selectivity. ‘These biomolecules have many different functional groups, so it’s not straightforward to selectively modify one of them’, says Kevin Neumann, assistant professor at Radboud University (RU). ‘In addition to selecting the targeted functionality, controlling the site of modification would be highly advantageous. Ultimately, we want to attach cytotoxic drug molecules to something, so ideally we want to know exactly which sites are covered and how many of them there are.’
This is why most labelling strategies focus on cysteines, explains Katerina Gavriel, a PhD student in Neumann’s precision chemistry group. ‘Cysteines are typically rare in proteins and are also highly nucleophilic at lower pH values. This means that, when competing lysine groups are inactive, cysteines remain available for modification.’
Surprise
The present project had an unexpected start. ‘We had a student, Daniëlle Geers, who had a few weeks left on her initial project’, says Gavriel. ‘While we were working hard on a tetrazine modification, we let her try similar chemistry with triazines.’ To their surprise, it worked really well and offered new reactivity. ‘We didn’t foresee that her initial findings would result in such a large and exciting project’, concurs Neumann.
Previously, researchers knew what kind of entities they could modify, but often these approaches were lacking site-selectivity, i.e. having control over where reactions occur. In class, Neumann usually uses the illustration of a box containing three apples and two bananas to explain this. ‘Taking an apple instead of a banana would be chemoselective, but we want to choose one of the three apples, symbolising site selectivity.’
‘Others have achieved site selectivity on the N-terminus of a protein, but not on the backbone — at least not selectively, without the need for protecting groups’, adds Gavriel. This is precisely what she and her colleagues achieved, as demonstrated in their publication in ChemistryEurope. Depending on the pH, the team could modify either the cysteine at the N-terminus or the backbone of the protein using triazine-thiol exchange.
Teaming up
However, the mechanism of selectivity was unclear, Gavriel explains. ‘Our biggest challenge was establishing the mechanism of selectivity. We observed that it happened, but we wanted to know more.’ The Neumann group turned to Jan Meisner, a professor at Heinrich Heine University, who helped them with computational studies to complement their NMR assays. ‘It shows why it’s always worthwhile for computational chemists and experimentalists to team up’, says Neumann.
Another challenge was more practical. Gavriel says that reducing the reactivity of the heterocycle to increase selectivity slowed down the reaction rate. ‘There was competition between our reaction and disulfide formation, which is something we still need to overcome.’ The group is actively working on finding the right balance between control and speed.
Nevertheless, Neumann continues, this is a ‘great fundamental achievement the first time around’. ‘This will definitely be applied to drug conjugates. For some applications, such control is essential; for example, for antibody drug conjugates. We were able to translate the research from small molecules all the way up to large peptides.’ Another benefit of this level of control relates to policy. ‘The FDA wants to know what they’re approving. If you can tell them which cysteine your modification is present on, that makes the approval process much faster, which is interesting for pharmaceutical companies. In other words, it’s time to team up with larger companies to really get this thing going.’
Gavriel, K. et al. (2025) ChemistryEurope 3(5), DOI: 10.1002/ceur.202500273












Nog geen opmerkingen