If you want to generate phosphorescence of organic molecules in solution, very precise tuning of the energy levels of the excited states of your molecule is required. In Chemistry - A European Journal, Flemish researchers shed light on this phenomenon.
Phosphorescence is a so-called ‘forbidden transition’; something you should not observe according to quantum chemistry rules. It pertains to the relapse of a molecule to a specific excited state, the triplet state. Normally speaking, an excited molecule enters the singlet state and then relapses while emitting light (fluorescence) or not.
So, the transition from singlet to triplet and the resulting phosphorescence - especially at room temperature - really shouldn’t occur. ‘But if you adjust your material in the right way, it can happen,’ says Wouter Maes, professor of organic synthesis at Hasselt University. His research group, together with colleagues from Durham University and Namen University, figured out how to get room-temperature phosphorescence of organic molecules in solution (sRTP) without the presence of heavy metals, which is very rare.
Small effect
‘It actually started like so many discoveries,’ says Maes. ‘In previous research, we accidentally stumbled upon the fact that the molecules we made in solution exhibit phosphorescence. In this paper, we study how that arises.’ For practical applications, this finding is far from relevant. ‘It remains a small effect, but it is fundamentally very interesting, precisely because it is such a rare phenomenon.’
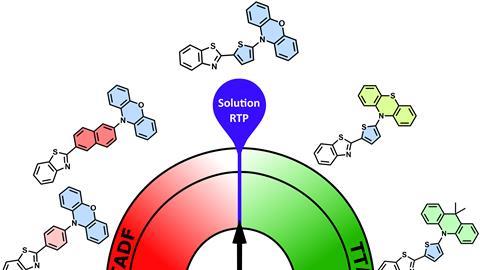
Maes’ group normally works with materials suitable for organic electronics such as OLEDs. In that case, the aim is to create more fluorescence, and one way to achieve that is through thermally activated delayed fluorescence (TADF). ‘You have to play around with the singlet and triplet levels, so that they get closer together and the transition from singlet to triplet and vice versa becomes easier. But when you start playing with those energy levels, you sometimes encounter other things, like in this case, sRTP.’
Energy gap
By matching different electron donors to the same acceptor and comparing them to the previously reported molecule, Maes and colleagues were able to determine which properties cause sRTP. ‘In doing so, we also felt it was important to rationally consider in advance - using DFT calculations - what the effects of the donors would be,’ Maes says.
Because the acceptor remained the same, the excited triplet state also remained the same, but the singlet state changed for each donor. Thus, in the original donor (phenoxazine), they saw phosphorescence. In the other donors, the distance to the singlet state was either too large (leading to triplet-triplet annihilation) or too small (leading to TADF). The researchers conclude that an energy gap of about 0.4 eV is optimal for phosphorescence to occur.












Nog geen opmerkingen