
The Nobel Prize in Chemistry has been awarded to Moungi Bawendi, Louis Brus and Alexei Ekimov for the discovery and synthesis of quantum dots.
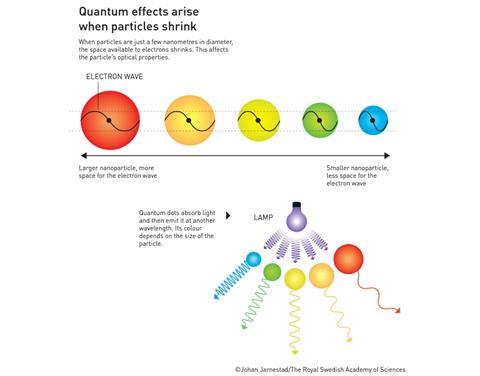
Quantum dots are a unique type of particle: their properties - think optical, catalytic, thermal or electrical - can be varied by changing their size. The theory behind this was already known in the 1930s, but it was not until the 1980s that Alexei Ekimov (Nanocrystals Technology Inc.) succeeded in creating size-dependent quantum effects in coloured glass. The size of the copper chloride particles used allowed Ekimov to change the colour.
A few years later, Louis Brus (Columbia University) was the first to demonstrate size-dependent quantum effects for particles suspended in a liquid. In 1993, Moungi Bawendi (MIT) succeeded in modifying the production method to produce near-perfect particles, opening the way to applications.
Aldo Brinkman, a chemist and science educator at the QuTech Institute, says on the phone: ‘Yes! Nice, absolutely fantastic.’ Brinkman explains that the discovery of quantum dots eventually led to the technique used by the institute itself, although these are slightly different quantum dots. ’In layman’s terms, quantum dots are dimensionless particles. They contain an electron. Because they are so small [nano-sized, ed], they lose their bulk properties and start to exhibit quantum properties’.
The oldest application dates back to the Middle Ages. ’Some stained glass windows use this principle’, explains Brinkman. ’If you grind gold very finely, the size of the gold beads can determine the colour of the glass you mix it into. Nowadays you can see the application in QLED screens: the quantum dots in these screens are more true to colour than the crystals in current LCD screens.’












Nog geen opmerkingen