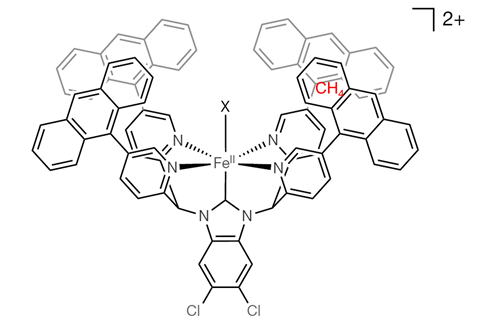
You can ‘hide’ methane in a cavity of an iron catalyst decorated with an organic structure and thus selectively oxidise it to methanol, according to a publication in Nature.
Methane is difficult to oxidise because of the high dissociation energy of its C–H bonds. If you want to break them, you have to use chemical tricks. Researchers at the University of Tsukuba in Japan have developed an iron catalyst that isolates a methane molecule in a hydrophobic cavity, selectively oxidises it and then repels the resulting methanol molecule.
The catalyst has several advantages. Usually catalysts are made from expensive or rare metals, but this one is made from iron, which is abundant and cheap. It is also soluble in water, which makes the reaction conditions very mild. The way it’s made helps to capture methane: the iron ion is in a hexagonal bipyramidal orientation, with large flat aromatic structures on the sides. At the top there’s an opening for water molecules to enter, and between the aromatic (and hydrophobic) structures there’s room for methane molecules. The researchers ‘anticipate that further development of this strategy might result in efficient and selective catalytic processes that can use naturally abundant carbon feedstocks.’

But they’re not there yet. ‘The setup they used is a tiny 10 ml reactor’, says Morteza Hadian, a PhD student at the TU Eindhoven who is working on a scale-up project at ARC CBBC. ‘It looks interesting, but these are just baby steps. There is still a lot to learn about this process.’
The first thing to do to adapt the Japanese setup for industrial purposes is to switch to a continuous process. ‘In the paper, they used a magnetic stirrer and a pressurised stream of alkane gas in a batch process’, Hadian explains. ‘So what they need instead is a different type of reactor, perhaps a bubbly flow system, and a way to introduce continuity into the system of one of the phases, ideally both.’
The researchers talk about a turnover number of 500 and a methanol selectivity of 83% in 3 hours. They say these are some of the highest figures ever reported for methane oxidation using a metal complex catalyst. Hadian isn’t really impressed. ‘If you want to make this on an industrial scale, you want to waste as little as possible. The selectivity is good, but there may still be room for improvement; you also need a high methane conversion. The product should be very valuable if you want to use these small amounts.’
However, the process certainly has potential. ‘An organic-iron catalyst would be very interesting on an industrial scale’, Hadian says. ‘In industry, many catalysts are made with precious metals for good reason, because they are super active and can last for months. Often the high value of the product outweighs the cost of these catalysts. But you wouldn’t want to use them for simple alcohols. So it would be promising to be able to oxidise methane with a cheap and relatively easy to produce iron catalyst.’
Fujisaki, H. et al. (2023) Nature, doi.org/10.1038/s41586-023-05821-2












Nog geen opmerkingen