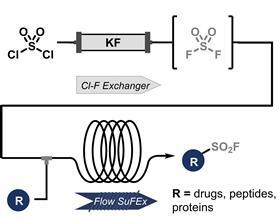
In a flow system, sulphuryl fluoride can be attached to drug precursors, peptides and proteins quickly, safely and efficiently, report researchers from Amsterdam in Nature Synthesis. They offer a convincing method for sulphur(VI) fluoride exchange click chemistry.
Click chemistry needs little introduction. The classic copper-catalysed coupling of an azide to an alkyn led to that year’s Nobel Prize. But this classic reaction was not the end of the story. Chemists have continued to develop the principle of click chemistry, and one of the successful reactions is the so-called sulphur(VI) fluoride exchange reaction (SuFEx), in which sulphuryl fluoride (SO2F2) is coupled to a molecule. However, sulphuryl fluoride itself is a toxic gas and is therefore avoided by many. Miguel Bernús, Daniella Mazzarella, Timothy Noël and colleagues at the University of Amsterdam have circumvented this problem and greatly increased the speed of the reaction by using flow chemistry.
Breakthrough
‘For me, this is a real breakthrough in SuFEx click chemistry’, says flow chemistry professor Noël. ‘We produce SO2F2 in situ on demand, just enough to complete the reaction. The HF released in the process is immediately quenched by the base present, and because the flow system remains closed, no gas escapes into the environment.’ Thus the safety risk is eliminated.
But the fact that SO2F2 can now be produced more safely is not the most remarkable thing about the study. ‘First, we coupled small, drug-like molecules to the sulphuryl group’, Noël continues. ‘This was so fast that the reaction was completed within two minutes.’ Most of the reactions yielded more than 80%. ‘Then we thought: If it’s this fast, why not try coupling with peptides? And again, the reaction was done in two minutes.’ The coupling is quite selective for the amino acid tyrosine, which is not entirely surprising given the benzene ring that is often present in drugs.
’I know of almost no other method that can do this so quickly and efficiently’
‘If you’ve come this far, you have to keep going’, says Noël. ‘The ultimate goal would be for our method to work on proteins, which are the most difficult to functionalise.’ And here, too, the results don’t lie: in ninety seconds, they managed to coat β-casein and myoglobin with sulphuryl fluoride. ‘I know of almost no other method that can do this so quickly and efficiently.’
There are other advantages to the flow method. For example, the researchers produced SO2F2 from the commercially available and inexpensive reagents SO2Cl2 and KF, which react in a separate chamber in the flow system. ‘The resulting SO2F2 is partly dissolved and partly in the form of small bubbles’, explains Noël. ‘So you shouldn’t make too much, 1.1 equivalents is enough.’ You need much less than in a round-bottom flask, where you would have to fill more space, partly because the SO2F2 ‘leaks’ to the top. ‘Our method also produces little to no fluorine waste.’
Scaling up
The potential for up-scaling is certainly there. Noël: ‘The current method bridges the gap between milligrams and a few grams, enough material to do evaluations. If you want to go even bigger, you can think about running several reactors in parallel or enlarging the reactors. With some adjustments in the size of the tubes, the kilogram scale should also be possible.’ Noël and his group are busy with follow-up research, but he is reluctant to reveal too much. ‘I am still a bit cautious, but it has to do with automation.’
Bernús, M., Mazzarella, D. et al. (2023) Nat. Synth., DOI: 10.1038/s44160-023-00441-0












Nog geen opmerkingen