Two papers from a group in Amsterdam show insights in electrochemical pathways. ‘If you want to transfer these processes towards the industrial scale, you need to understand the pathway.’
Electrochemistry is often portrayed as green compared to traditional organic or catalytic chemistry. But what makes it green? ‘When you talk about the energy transition in particular, it is green in the sense that you can drive electrochemical reactions with just electrons’, says Amanda Garcia, assistant professor at the University of Amsterdam. ‘You don’t need high temperature or pressure, and you can usually do the reactions in water, although we’re also looking at friendlier organic solvents.’ One caveat, of course, is that the electrons should be green, generated by wind turbines or solar panels.
Recently, Garcia’s group published two papers, in the Journal of the American Chemical Society and ChemCatChem, on synthesising useful compounds in a greener and more sustainable way. ‘Although they are quite different in terms of content, all the reactions are driven by electrons under ambient conditions.’
Cheap
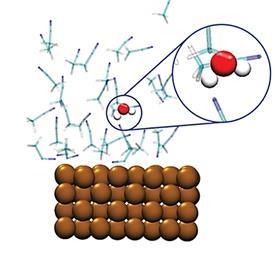
In ChemCatChem, Garcia and her team shed light on a common problem with the CO2 reduction reaction (CRR). ‘The energy you put into the system is partly consumed by a parasitic reaction to produce hydrogen’, says Garcia. The problem arose when they started working with organic solvents, which changed the reaction mechanism of the CRR. ‘Organic solvents always contain a very small amount of water. These few parts per million are enough to change the reaction mechanism towards the hydrogen product. We ran some simulations to see at what point this mechanism becomes dominant.’ It turns out that water has a preferential interaction with the copper electrode, more so than the acetonitrile they used. It therefore seems essential to dry your solvents very well.
The main idea proposed in JACS is the electrochemical formation of carbon-nitrogen bonds. ‘We were curious to see if this was possible and decided to try to make formamides’, explains Garcia. ‘These types of compounds have many applications, and until now it has been very difficult to make them without metal catalysts. Here we show that you can do it with a cheap glassy carbon electrode.’
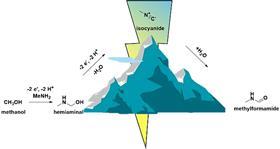
‘A big surprise was that we were able to make methyl isocyanide, a very toxic chemical, safely,’ Garcia continues. ‘That’s why it was accepted in JACS. One of the committee members said to us: “You’ve found a very cheap and simple mechanism to do exactly what I was looking for!” Even though I wasn’t sure I wanted to shoot for this journal, Ph.D. student Pim Broersen, who designed the experiment, was absolutely convinced to try it, and he was right.’
Inaccessible
In this way, electrochemistry opens up the possibility of synthesising valuable chemicals in ways that are often inaccessible via traditional organic or catalytic chemistry. But why does it work? In both papers, Garcia’s team used advanced electrochemical methods to investigate the mechanism behind the electrochemical reactions. ‘We use in situ spectroscopic techniques to identify the key intermediates, which are often stable radicals. Once you know what’s forming in the middle of the process, it becomes easier to design other reactions.’
It is a crucial step that is often overlooked. ‘People often just look at the end product, which is fine, but if you want to transfer these processes to industrial scale, you need to understand the pathway to increase the yield.’
Electrochemistry is ideal for this because it offers a lot of control. ‘To tune a reaction, you can adjust the current or voltage. That’s much more flexible than, say, tuning the pressure; many industrial processes already operate at high pressures, which are difficult to tune further. But electrochemistry is different, it’s very sensitive and you can control exactly how much charge goes through the system.’
Broersen, P.J.L. et al. (2025) JACS 147(12), DOI: 10.1021/jacs.4c16725
Deacon-Price, C. et al. (2025) ChemCatChem 17(6), DOI: 10.1002/cctc.202401332


















Nog geen opmerkingen