The model behind hydrogen bonds and similar complexes is fundamentally flawed, Amsterdam researchers show in Chemistry – A European Journal.
Most everyone familiar with chemical concepts will recognise the hydrogen bond, a pretty strong intermolecular interaction between a hydrogen atom and for example oxygen, nitrogen or halogens (think of the base pairs in DNA). For most people, the idea behind this is that hydrogen is electropositive and thus has a relatively positive charge. The other molecule would contain electronegative atoms and thus have a relatively negative charge. This will cause an electrostatic attraction, similar to what occurs in magnets.
’Knowing the fundamental physics makes you understand nature better’
‘However, problems arose when people realised that two electronegative atoms could also attract one another’, says Lucas de Azevedo Santos, postdoc at the TheoCheM group at the Vrije Universiteit Amsterdam. You’ll find this phenomenon in so-called pnictogen, chalcogen and halogen bonds, which are Lewis acid-base interactions between group 15, 16 and 17 elements, respectively. ‘To explain this, the σ- (sigma) hole model was postulated. This model looks at the bond donating Lewis acid as having a positive region on the surface around the molecule, while the bond accepting Lewis base is treated like a concentrated negative point charge.’ But new calculations using molecular orbital (MO) theory shows that this model neglects two very important aspects: the physics behind both electrostatic interactions and the directionality of intermolecular interactions.
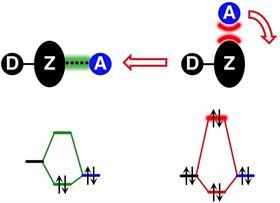
‘Mind you, the σ-hole does exist, as it has been experimentally detected’, says De Azevedo Santos. ‘But the point is that there is no causal relationship between the σ-hole and the existence and formation of intermolecular complexes.’ To prove their point, De Azevedo Santos and his colleagues did quantum chemical calculations with FmZ∙∙∙F–, where m = 1-3 and Z is a pnictogen, chalcogen or halogen atom.
If the σ-hole model is correct, you would expect that the bond strength and directionality are determined by the attraction of the positive region and the negative point charge, but that’s not the case. This model cannot explain the physics that should follow from it. As the authors wrote in the paper: ‘The reason for this behaviour is simple: molecules are not electrostatic potentials on surfaces and atoms are not point charges!’ De Azevedo Santos: ‘Molecules are made up of atoms with a positive nucleus and a negatively charged electron density surrounding it. The σ-hole model treats the molecules physically wrong.’
But the authors did not only criticize the model, they postulated their own explanation in the paper too. ‘We propose that the basis for these interactions are “intermolecular covalent interactions”. All these bonds and complexes share electrons!’ the postdoc explains. ‘Technically speaking, these bonds are made up of an empty orbital – the σ* LUMO as electron acceptor – and a filled orbital – the HOMO. These orbitals overlap in the pnictogen, chalcogen and halogen bond interactions, which causes bonding.’
But why should we care about this? ‘Because knowing the fundamental physics makes you understand nature better’, De Azevedo Santos says. ‘If you understand the interactions, you can use this knowledge for the rational design of molecules. For example, there are biological and synthetic systems with the function of transporting anions across lipid membranes. These systems use those specific interactions. So, if you know how they work, you can improve the activity specifically for this transporter.’
De Azevedo Santos, L. et al. (2023) Chem. Eur. J. e202203791, DOI: 10.1002/chem.202203791












Nog geen opmerkingen